Abstract
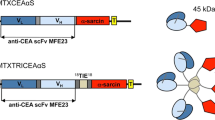
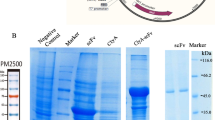
This work describes use of anti-carcinoembryonic antigen antibodies (10H6, T84.66) for targeted delivery of an endosomal escape peptide (H6CM18) and gelonin, a type I ribosome inactivating protein. The viability of colorectal cancer cells (LS174T, LoVo) was assessed following treatment with gelonin or gelonin immunotoxins, with or without co-treatment with T84.66-H6CM18. Fluorescent microscopy was used to visualize the escape of immunoconjugates from endosomes of treated cells, and efficacy and toxicity were assessed in vivo in xenograft tumor-bearing mice following single- and multiple-dose regimens. Application of 25 pM T84.66-H6CM18 combined with T84.66-gelonin increased gelonin potency by ~ 1,000-fold and by ~ 6,000-fold in LS174T and LoVo cells. Intravenous 10H6-gelonin at 1.0 mg/kg was well tolerated by LS174T tumor-bearing mice, while 10 and 25 mg/kg doses led to signs of toxicity. Single-dose administration of PBS, gelonin conjugated to T84.66 or 10H6, T84.66-H6CM18, or gelonin immunotoxins co-administered with T84.66-H6CM18 were evaluated. The combinations of T84.66-gelonin + 1.0 mg/kg T84.66-H6CM18 and 10H6-gelonin + 0.1 mg/kg T84.66-H6CM18 led to significant delays in LS174T growth. Use of a multiple-dose regimen allowed further anti-tumor effects, significantly extending median survival time by 33% and by 69%, for mice receiving 1 mg/kg 10H6-gelonin + 0.1 mg/kg T84.66-H6CM18 (p = 0.0072) and 1 mg/kg 10H6-gelonin + 1 mg/kg T84.66-H6CM18 (p = 0.0017). Combined administration of gelonin immunoconjugates with antibody-targeted endosomal escape peptides increased the delivery of gelonin to the cytoplasm of targeted cells, increased gelonin cell killing in vitro by 1,000–6,000 fold, and significantly increased in vivo efficacy.
Graphical Abstract







Similar content being viewed by others
References
FitzGerald DJ, Kreitman R, Wilson W, Squires D, Pastan I. Recombinant immunotoxins for treating cancer. Int J Med Microbiol. 2004;293(7–8):577–82.
Kreitman RJ. Recombinant immunotoxins for the treatment of chemoresistant hematologic malignancies. Curr Pharm Des. 2009;15(23):2652–64.
Alewine C, Hassan R, Pastan I. Advances in anticancer immunotoxin therapy. Oncologist. 2015;20(2):176–85.
Frankel AE, Houston LL, Issell BF, Fathman G. Prospects for immunotoxin therapy in cancer. Annu Rev Med. 1986;37:125–42.
Walsh MJ, Dodd JE, Hautbergue GM. Ribosome-inactivating proteins: potent poisons and molecular tools. Virulence. 2013;4(8):774–84.
de Virgilio M, Lombardi A, Caliandro R, Fabbrini MS. Ribosome-inactivating proteins: from plant defense to tumor attack. Toxins (Basel). 2010;2(11):2699–737.
Eiklid K, Olsnes S, Pihl A. Entry of lethal doses of abrin, ricin and modeccin into the cytosol of HeLa cells. Exp Cell Res. 1980;126(2):321–6.
Lambert JM, Blattler WA, McIntyre GD, Goldmacher VS, Scott CF. Immunotoxins containing single chain ribosome-inactivating proteins. In: Frankel AE, editor. Immunotoxins: Kluwer Academic Publishers; 1988. p. 180.
Taylor NS, Pollack M. Purification of Pseudomonas aeruginosa exotoxin by affinity chromatography. Infect Immun. 1978;19(1):66–70.
Lyu MA, Cao YJ, Mohamedali KA, Rosenblum MG. Cell-targeting fusion constructs containing recombinant gelonin. Methods Enzymol. 2012;502:167–214.
Borthakur G, Rosenblum MG, Talpaz M, Daver N, Ravandi F, Faderl S, et al. Phase 1 study of an anti-CD33 immunotoxin, humanized monoclonal antibody M195 conjugated to recombinant gelonin (HUM-195/rGEL), in patients with advanced myeloid malignancies. Haematologica. 2013;98(2):217–21.
Pirie CM, Hackel BJ, Rosenblum MG, Wittrup KD. Convergent potency of internalized gelonin immunotoxins across varied cell lines, antigens, and targeting moieties. J Biol Chem. 2011;286(6):4165–72.
Yazdi PT, Murphy RM. Quantitative analysis of protein synthesis inhibition by transferrin-toxin conjugates. Cancer Res. 1994;54(24):6387–94.
Copolovici DM, Langel K, Eriste E, Langel U. Cell-penetrating peptides: design, synthesis, and applications. ACS Nano. 2014;8(3):1972–94.
Guidotti G, Brambilla L, Rossi D. Cell-Penetrating Peptides: From Basic Research to Clinics. Trends Pharmacol Sci. 2017;38(4):406–24.
Haas DH, Murphy RM. Design of a pH-sensitive pore-forming peptide with improved performance. J Pept Res. 2004;63(1):9–16.
Li W, Nicol F, Szoka FC Jr. GALA: a designed synthetic pH-responsive amphipathic peptide with applications in drug and gene delivery. Adv Drug Deliv Rev. 2004;56(7):967–85.
Parente RA, Nir S, Szoka FC Jr. Mechanism of leakage of phospholipid vesicle contents induced by the peptide GALA. Biochemistry. 1990;29(37):8720–8.
Junghans R, Waldmann T. Metabolism of Tac (IL2Ralpha): physiology of cell surface shedding and renal catabolism, and suppression of catabolism by antibody binding. J Exp Med. 1996;183(4):1587–602.
Igawa T, Ishii S, Tachibana T, Maeda A, Higuchi Y, Shimaoka S, et al. Antibody recycling by engineered pH-dependent antigen binding improves the duration of antigen neutralization. Nat Biotechnol. 2010;28(11):1203–7.
Chaparro-Riggers J, Liang H, Devay RM, Bai L, Sutton JE, Chen W, et al. Increasing Serum Half-life and Extending Cholesterol Lowering in vivo by Engineering Antibody with pH-sensitive Binding to PCSK9. J Biol Chem. 2012;287(14):11090–7.
Engler FA, Polli JR, Li T, An B, Otteneder M, Qu J, et al. “Catch-and-Release” Anti-Carcinoembryonic Antigen Monoclonal Antibody Leads to Greater Plasma and Tumor Exposure in a Mouse Model of Colorectal Cancer. J Pharmacol Exp Ther. 2018;366(1):205–19.
Shi ZR, Tsao D, Kim YS. Subcellular distribution, synthesis, and release of carcinoembryonic antigen in cultured human colon adenocarcinoma cell lines. Cancer Res. 1983;43(9):4045–9.
Levin LV, Griffin TW, Childs LR, Davis S, Haagensen DE Jr. Comparison of multiple anti-CEA immunotoxins active against human adenocarcinoma cells. Cancer Immunol Immunother. 1987;24(3):202–6.
Hermanson GT. Bioconjugate techniques. Third edition. ed. London ; Waltham, MA: Elsevier/AP; 2013. xvii, 1146 pages p.
Engler FA, Balthasar JP. Development and validation of an enzyme-linked immunosorbent assay for the quantification of gelonin in mouse plasma. J Immunoassay Immunochem. 2016;37(6):611–22.
Eeftens JM, van der Torre J, Burnham DR, Dekker C. Copper-free click chemistry for attachment of biomolecules in magnetic tweezers. BMC Biophys. 2015;8:9.
Dunn KW, Kamocka MM, McDonald JH. A practical guide to evaluating colocalization in biological microscopy. Am J Physiol Cell Physiol. 2011;300(4):C723–42.
Bolte S, Cordelières FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224(Pt 3):213–32.
Salomone F, Breton M, Leray I, Cardarelli F, Boccardi C, Bonhenry D, et al. High-yield nontoxic gene transfer through conjugation of the CM18-Tat11 chimeric peptide with nanosecond electric pulses. Mol Pharm. 2014;11(7):2466–74.
Salomone F, Cardarelli F, Di Luca M, Boccardi C, Nifosì R, Bardi G, et al. A novel chimeric cell-penetrating peptide with membrane-disruptive properties for efficient endosomal escape. J Control Release. 2012;163(3):293–303.
Del’Guidice T, Lepetit-Stoffaes JP, Bordeleau LJ, Roberge J, Théberge V, Lauvaux C, et al. Membrane permeabilizing amphiphilic peptide delivers recombinant transcription factor and CRISPR-Cas9/Cpf1 ribonucleoproteins in hard-to-modify cells. PLoS One. 2018;13(4):e0195558.
King HD, Dubowchik GM, Mastalerz H, Willner D, Hofstead SJ, Firestone RA, et al. Monoclonal antibody conjugates of doxorubicin prepared with branched peptide linkers: inhibition of aggregation by methoxytriethyleneglycol chains. J Med Chem. 2002;45(19):4336–43.
Oyama K, Takabayashi M, Takei Y, Arai S, Takeoka S, Ishiwata S, et al. Walking nanothermometers: spatiotemporal temperature measurement of transported acidic organelles in single living cells. Lab Chip. 2012;12(9):1591–3.
Shi B, Huang Q-Q, Birkett R, Doyle R, Dorfleutner A, Stehlik C, et al. SNAPIN is critical for lysosomal acidification and autophagosome maturation in macrophages. Autophagy. 2017;13(2):285–301.
Scott CF Jr, Goldmacher VS, Lambert JM, Chari RV, Bolender S, Gauthier MN, et al. The antileukemic efficacy of an immunotoxin composed of a monoclonal anti-Thy-1 antibody disulfide linked to the ribosome-inactivating protein gelonin. Cancer Immunol Immunother. 1987;25(1):31–40.
Scott CF Jr, Lambert JM, Goldmacher VS, Blatter WA, Sobel R, Schlossman SF, et al. The pharmacokinetics and toxicity of murine monoclonal antibodies and of gelonin conjugates of these antibodies. Int J Immunopharmacol. 1987;9(2):211–25.
Moolten FL, Cooperband SR. Selective destruction of target cells by diphtheria toxin conjugated to antibody directed against antigens on the cells. Science. 1970;169(3940):68–70.
Pastan I, Hassan R, FitzGerald DJ, Kreitman RJ. Immunotoxin therapy of cancer. Nat Rev Cancer. 2006;6(7):559–65.
Akbari B, Farajnia S, AhdiKhosroshahi S, Safari F, Yousefi M, Dariushnejad H, et al. Immunotoxins in cancer therapy: Review and update. Int Rev Immunol. 2017;36(4):207–19.
Bordeau BM, Balthasar JP. Strategies to enhance monoclonal antibody uptake and distribution in solid tumors. Cancer Biol Med. 2021;18(3):649–64.
Shan L, Liu Y, Wang P. Recombinant Immunotoxin Therapy of Solid Tumors: Challenges and Strategies. J Basic Clin Med. 2013;2(2):1–6.
Pastan I, Hassan R, FitzGerald DJ, Kreitman RJ. Immunotoxin Treatment of Cancer. Annu Rev Med. 2007;58(1):221–37.
Posey JA, Khazaeli MB, Bookman MA, Nowrouzi A, Grizzle WE, Thornton J, et al. A Phase I Trial of the Single-Chain Immunotoxin SGN-10 (BR96 sFv-PE40) in Patients with Advanced Solid Tumors. Clin Cancer Res. 2002;8(10):3092–9.
Marcil J, Ravindranath N, Sairam MR. Cytotoxic activity of lutropin-gelonin conjugate in mouse Leydig tumor cells: Potentiation of the hormonotoxin activity by different drugs. Mol Cell Endocrinol. 1993;92(1):83–90.
Mujoo K, Reisfeld RA, Cheung L, Rosenblum MG. A potent and specific immunotoxin for tumor cells expressing disialoganglioside GD2. Cancer Immunol Immunother. 1991;34(3):198–204.
Davol PA, Bizuneh A, Frackelton AR. Wortmannin, a phosphoinositide 3-kinase inhibitor, selectively enhances cytotoxicity of receptor-directed-toxin chimeras in vitro and in vivo. Anticancer Res. 1999;19(3A):1705–13.
Shin MC, Zhao J, Zhang J, Huang Y, He H, Wang M, et al. Recombinant TAT-gelonin fusion toxin: synthesis and characterization of heparin/protamine-regulated cell transduction. J Biomed Mater Res A. 2015;103(1):409–19.
Berstad MB, Cheung LH, Berg K, Peng Q, Fremstedal ASV, Patzke S, et al. Design of an EGFR-targeting toxin for photochemical delivery: in vitro and in vivo selectivity and efficacy. Oncogene. 2015;34(44):5582–92.
Berg K, Høgset A, Prasmickaite L, Weyergang A, Bonsted A, Dietze A, et al. Photochemical internalization (PCI): A novel technology for activation of endocytosed therapeutic agents. Med Laser Appl. 2006;21(4):239–50.
Dietze A, Peng Q, Selbo PK, Kaalhus O, Müller C, Bown S, et al. Enhanced photodynamic destruction of a transplantable fibrosarcoma using photochemical internalisation of gelonin. Br J Cancer. 2005;92(11):2004–9.
Selbo PK, Sivam G, Fodstad Ø, Sandvig K, Berg K. In vivo documentation of photochemical internalization, a novel approach to site specific cancer therapy. Int J Cancer. 2001;92(5):761–6.
Bull-Hansen B, Berstad MB, Berg K, Cao Y, Skarpen E, Fremstedal AS, et al. Photochemical activation of MH3-B1/rGel: a HER2-targeted treatment approach for ovarian cancer. Oncotarget. 2015;6(14):12436–51.
Weyergang A, Fremstedal AS, Skarpen E, Peng Q, Mohamedali KA, Eng MS, et al. Light-enhanced VEGF121/rGel: A tumor targeted modality with vascular and immune-mediated efficacy. J Control Release. 2018;288:161–72.
Shin MC, Zhang J, Min KA, He H, David AE, Huang Y, et al. PTD-Modified ATTEMPTS for Enhanced Toxin-based Cancer Therapy: An In vivo Proof-of-Concept Study. Pharm Res. 2015;32(8):2690–703.
Pirie CM, Liu DV, Wittrup KD. Targeted cytolysins synergistically potentiate cytoplasmic delivery of gelonin immunotoxin. Mol Cancer Ther. 2013;12(9):1774–82.
Salomone F, Breton M, Leray I, Cardarelli F, Boccardi C, Bonhenry D, et al. High-yield nontoxic gene transfer through conjugation of the CM(1)(8)-Tat(1)(1) chimeric peptide with nanosecond electric pulses. Mol Pharm. 2014;11(7):2466–74.
Salomone F, Cardarelli F, Di Luca M, Boccardi C, Nifosi R, Bardi G, et al. A novel chimeric cell-penetrating peptide with membrane-disruptive properties for efficient endosomal escape. J Control Release. 2012;163(3):293–303.
Del Guidice T, Lepetit-Stoffaes JP, Bordeleau LJ, Roberge J, Theberge V, Lauvaux C, et al. Membrane permeabilizing amphiphilic peptide delivers recombinant transcription factor and CRISPR-Cas9/Cpf1 ribonucleoproteins in hard-to-modify cells. Plos One. 2018;13(4).
Dubowchik GM, Firestone RA, Padilla L, Willner D, Hofstead SJ, Mosure K, et al. Cathepsin B-labile dipeptide linkers for lysosomal release of doxorubicin from internalizing immunoconjugates: model studies of enzymatic drug release and antigen-specific in vitro anticancer activity. Bioconjug Chem. 2002;13(4):855–69.
Dubowchik GM, Mosure K, Knipe JO, Firestone RA. Cathepsin B-sensitive dipeptide prodrugs. 2. Models of anticancer drugs paclitaxel (Taxol), mitomycin C and doxorubicin. Bioorg Med Chem Lett. 1998;8(23):3347–52.
Abuqayyas L, Balthasar JP. Application of PBPK modeling to predict monoclonal antibody disposition in plasma and tissues in mouse models of human colorectal cancer. J Pharmacokinet Pharmacodyn. 2012;39(6):683–710.
Mujoo K, Cheung L, Murray JL, Rosenblum MG. Pharmacokinetics, tissue distribution, and in vivo antitumor effects of the antimelanoma immunotoxin ZME-gelonin. Cancer Immunol Immunother. 1995;40(5):339–45.
Fujimori K, Covell DG, Fletcher JE, Weinstein JN. A modeling analysis of monoclonal antibody percolation through tumors: a binding-site barrier. J Nucl Med. 1990;31(7):1191–8.
Bordeau BM, Yang Y, Balthasar JP. Transient Competitive Inhibition Bypasses the Binding Site Barrier to Improve Tumor Penetration of Trastuzumab and Enhance T-DM1 Efficacy. Cancer Res. 2021;81(15):4145–54.
Bordeau BM, Abuqayyas L, Nguyen TD, Chen P, Balthasar JP. Development and Evaluation of Competitive Inhibitors of Trastuzumab-HER2 Binding to Bypass the Binding-Site Barrier. Front Pharmacol. 2022;13.
Caron PC, Jurcic JG, Scott AM, Finn RD, Divgi CR, Graham MC, et al. A phase 1B trial of humanized monoclonal antibody M195 (anti-CD33) in myeloid leukemia: specific targeting without immunogenicity. Blood. 1994;83(7):1760–8.
Urva SR, Yang VC, Balthasar JP. Physiologically based pharmacokinetic model for T84.66: a monoclonal anti-CEA antibody. J Pharm Sci. 2010;99(3):1582–600.
Shin MC, Zhang J, Ah Min K, Lee K, Moon C, Balthasar JP, et al. Combination of antibody targeting and PTD-mediated intracellular toxin delivery for colorectal cancer therapy. J Control Release. 2014;194:197–210.
Blumenthal RD, Osorio L, Hayes MK, Horak ID, Hansen HJ, Goldenberg DM. Carcinoembryonic antigen antibody inhibits lung metastasis and augments chemotherapy in a human colonic carcinoma xenograft. Cancer Immunol Immunother. 2005;54(4):315–27.
Vassileva V, Rajkumar V, Mazzantini M, Robson M, Badar A, Sharma S, et al. Significant Therapeutic Efficacy with Combined Radioimmunotherapy and Cetuximab in Preclinical Models of Colorectal Cancer. J Nucl Med. 2015;56(8):1239–45.
Kato J, Futamura M, Kanematsu M, Gaowa S, Mori R, Tanahashi T, et al. Combination therapy with zoledronic acid and cetuximab effectively suppresses growth of colorectal cancer cells regardless of KRAS status. Int J Cancer. 2016;138(6):1516–27.
Saridaki Z, Georgoulias V, Souglakos J. Mechanisms of resistance to anti-EGFR monoclonal antibody treatment in metastatic colorectal cancer. World J Gastroenterol. 2010;16(10):1177–87.
Acknowledgements
The authors would like to thank Jacobs School of Medicine and Biomedical Sciences for the use of Leica DMi 8 inverted fluorescence microscope for live cell imaging.
Funding
This work was supported by the National Cancer Institute of National Institutes of Health (grant numbers CA204192 and CA246785).
Author information
Authors and Affiliations
Contributions
J.R.P. and J.P.B. contributed to conceptualization; J.R.P. and P.C. contributed to methodology; J.R.P and P.C. contributed to investigation; J.R.P. contributed to writing—original draft preparation; J.R.P., P.C., B.M.B. and J.P.B. contributed to writing—review and editing; J.P.B. contributed to funding acquisition; J.P.B. provided the resources; J.P.B. contributed to supervision.
Corresponding author
Ethics declarations
Conflict of Interest Disclosure Statement
J.P.B. serves as the Director of the University at Buffalo Center for Protein Therapeutics, which is supported by an industry consortium. Through the consortium, J.P.B. has received research support, for work unrelated to this report, from Abbvie, Amgen, CSL-Behring, Eli Lilly, Genentech, GSK, Janssen, Merck, Roche, and Sanofi. During the course of this work, J.P.B. has received consulting fees from companies involved with the development of cancer therapies, including: Abbvie, Amgen, Eli Lilly, Merck, and Pfizer
Ethical Approval
Protocols for animal experiments were approved by the Institutional Animal Use and Care Committee of the University at Buffalo (PHC44086Y).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Polli, J.R., Chen, P., Bordeau, B.M. et al. Targeted Delivery of Endosomal Escape Peptides to Enhance Immunotoxin Potency and Anti-cancer Efficacy. AAPS J 24, 47 (2022). https://doi.org/10.1208/s12248-022-00698-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-022-00698-x