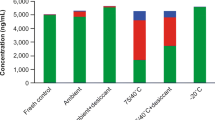
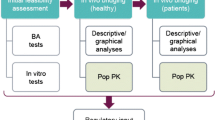
Abstract
In-clinic venous dried blood spot (DBS) pharmacokinetic (PK) sampling was incorporated into two phase 3 studies of verubecestat for Alzheimer’s disease (EPOCH [NCT01739348] and APECS [NCT01953601]), as a potential alternative to plasma PK sampling. Initially, plasma and DBS PK samples were collected concurrently to better understand the DBS–plasma verubecestat concentration relationship, with the intention of discontinuing DBS or plasma sampling following interim analysis. Following initial analyses and comparison of results with prespecified selection criteria, plasma PK sampling was discontinued; however, a stability issue resulting in generally lower DBS verubecestat concentrations with longer collection-to-assay times was subsequently discovered (associated with non-compliance in DBS sample handling), prompting reintroduction of plasma sampling. To enable inclusion of DBS data in population PK analyses, a conversion algorithm for calculating plasma-equivalent concentrations (accounting for DBS sample instability) was developed using paired (time-matched) plasma and DBS data from the EPOCH study. Verubecestat population PK models developed from pooled phase 1/1b and EPOCH data using either (1) plasma-only data or (2) plasma and plasma-equivalent concentrations (calculated from non-paired DBS samples) yielded similar results. The algorithm robustness was demonstrated using DBS data from paired samples from the APECS study and comparison between plasma and plasma-equivalent concentrations. The population PK model was updated with APECS data (both plasma and, if no plasma sample available, plasma equivalents). The results demonstrated similar PK in the two phase 3 populations and exposures consistent with expectations from phase 1 data. This case study illustrates challenges with employing new sampling techniques in large, global trials and describes lessons learned.
Graphical abstract







Similar content being viewed by others
References
Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8(6):595–608.
Scott JD, Li SW, Brunskill AP, Chen X, Cox K, Cumming JN, et al. Discovery of the 3-imino-1,2,4-thiadiazinane 1,1-dioxide derivative verubecestat (MK-8931)-a β-site amyloid precursor protein cleaving enzyme 1 inhibitor for the treatment of Alzheimer’s Disease. J Med Chem. 2016;59(23):10435–50.
Kennedy ME, Stamford AW, Chen X, Cox K, Cumming JN, Dockendorf MF, et al. The BACE1 inhibitor verubecestat (MK-8931) reduces CNS β-amyloid in animal models and in Alzheimer’s disease patients. Sci Transl Med. 2016;8(363):363ra150.
Min KC, Dockendorf MF, Palcza J, Tseng J, Ma L, Stone JA, et al. Pharmacokinetics and pharmacodynamics of the BACE1 inhibitor verubecestat (MK-8931) in healthy Japanese adults: a randomized, placebo-controlled study. Clin Pharmacol Ther. 2019;105(5):1234–43.
Forman M, Palcza J, Tseng J, Stone JA, Walker B, Swearingen D, Troyer MD, Dockendorf MF. Safety, tolerability, and pharmacokinetics of the beta-site amyloid precursor protein-cleaving enzyme 1 inhibitor verubecestat (MK-8931) in healthy elderly male and female subjects. Clin Transl Sci. 2019;12(5):545–55.
Egan MF, Kost J, Tariot PN, Aisen PS, Cummings JL, Vellas B, Sur C, Mukai Y, Voss T, Furtek C, Mahoney E, Harper Mozley L, Vandenberghe R, Mo Y, Michelson D. Randomized trial of verubecestat for mild-to-moderate Alzheimer’s disease. N Engl J Med. 2018;378(18):1691–703.
Egan MF, Kost J, Voss T, Mukai Y, Aisen PS, Cummings JL, Tariot PN, Vellas B, van Dyck CH, Boada M, Zhang Y, Li W, Furtek C, Mahoney E, Harper Mozley L, Mo Y, Sur C, Michelson D. Randomized trial of verubecestat for prodromal Alzheimer’s disease. N Engl J Med. 2019;380(18):1408–20.
Kothare PA, Bateman KP, Dockendorf M, Stone J, Xu Y, Woolf E, Shipley LA. An Integrated Strategy for Implementation of Dried Blood Spots in Clinical Development Programs. AAPS J. 2016;18(2):519–27.
Anderson M, Dockendorf MF, McIntosh I, Xie I, Breidinger S, Meng D, Ren S, Zhong W, Zhang L, Roadcap B, Bateman KP, Stone J, Woolf E. An investigation of instability in dried blood spot samples for pharmacokinetic sampling in phase 3 trials of verubecestat. AAPS J. 2022. In press.
Denniff P, Spooner N. The effect of hematocrit on assay bias when using DBS samples for the quantitative bioanalysis of drugs. Bioanalysis. 2010;2(8):1385–95.
Evans C, Arnold M, Bryan P, Duggan J, James CA, Li W, Lowes S, Matassa L, Olah T, Timmerman P, Wang X, Wickremsinhe E, Williams J, Woolf E, Zane P. Implementing dried blood spot sampling for clinical pharmacokinetic determinations: considerations from the IQ Consortium Microsampling Working Group. AAPS J. 2015;17(2):292–300.
Dockendorf MF, Murthy G, Bateman KP, Kothare PA, Anderson M, Xie I, Sachs JR, Burlage R, Goldman A, Moyer M, Shah JK, Ruba R, Shipley L, Harrelson J. Leveraging Digital Health Technologies and Outpatient Sampling in Clinical Drug Development: A Phase I Exploratory Study. Clin Pharmacol Ther. 2019;105(1):168–76.
Li CC, Dockendorf M, Kowalski K, Yang B, Xu Y, Xie I, Kleijn HJ, Bosch R, Jones C, Thornton B, Marcantonio EE, Voss T, Bateman KP, Kothare PA. Population PK Analyses of Ubrogepant (MK-1602), a CGRP Receptor Antagonist: Enriching In-Clinic Plasma PK Sampling With Outpatient Dried Blood Spot Sampling. J Clin Pharmacol. 2018;58(3):294–303.
Dockendorf MF, Hansen BJ, Bateman KP, Moyer M, Shah JK, Shipley LA. Digitally Enabled, Patient-Centric Clinical Trials: Shifting the Drug Development Paradigm. Clin Transl Sci. 2021;14(2):445–59.
Acknowledgments
Medical writing assistance, under the direction of the authors, was provided by Kirsty Muirhead, PhD, of CMC AFFINITY, McCann Health Medical Communications, in accordance with Good Publication Practice (GPP3) guidelines. This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Funding
Funding for this research was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Author information
Authors and Affiliations
Contributions
Conception, design or planning of the study: MFD, TT, ND, CJ, CF, KPB, EW, MFE, JAS
Acquisition of the data: MA, CF, BK, MFE
Analysis of the data: MFD, DJ, RH, LM
Interpretation of the results: MFD, DJ, SB, LM, EW, MFE, JAS
Critically reviewing or revising the manuscript for important intellectual content: MFD, DJ, RH, MA, SB, LM, TT, ND, CJ, CF, BK, KPB, EW, MFE, JAS
Corresponding author
Ethics declarations
MFD, MA, SB, LM, TT, ND, CJ, CF, BK, KPB, EW, MFE, and JAS are current or former employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and may own stock and/or stock options in Merck & Co., Inc., Kenilworth, NJ, USA. DJ and RH are employees of Cognigen Corporation.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 600 kb)
Rights and permissions
About this article
Cite this article
Dockendorf, M.F., Jaworowicz, D., Humphrey, R. et al. A Model-Based Approach to Bridging Plasma and Dried Blood Spot Concentration Data for Phase 3 Verubecestat Trials. AAPS J 24, 53 (2022). https://doi.org/10.1208/s12248-022-00682-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-022-00682-5