Abstract
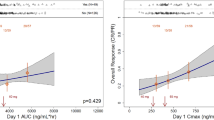
We developed an integrated population pharmacokinetic model to investigate loncastuximab tesirine pharmacokinetics (PK) and exposure–response relationships for relapsed/refractory B cell non-Hodgkin lymphoma, including diffuse large B cell lymphoma (DLBCL). The model, based on the recommended dosing schedule (150 µg/kg every 3 weeks [Q3W] for 2 cycles; 75 µg/kg Q3W thereafter) and drug concentrations in phase 1 and 2 studies (DLBCL [n = 284], non-DLBCL [n = 44]), was used to characterize loncastuximab tesirine PK and evaluate exposure covariates. Relationships between exposure (pyrrolobenzodiazepine-conjugated antibody [cAb] cycle 1 average concentration) and (1) efficacy (including overall response rate [ORR; primary endpoint] and overall survival [OS]) and (2) grade ≥ 2 treatment-emergent adverse events were explored. Statistical analyses included univariate and multivariate logistic regression, Kaplan–Meier analysis, and Cox proportional hazard regression. cAb and total Ab were best described by a two-compartment linear model with time-dependent clearance. The cAb steady-state half-life increased to 20.6 days by ~ 15 weeks. cAb exposure was lower for low albumin, mild/moderate hepatic impairment, non-DLBCL subtypes, and Eastern Cooperative Oncology Group scores > 1. Significant positive associations were reported between exposure and ORR (p = 3.21E-6), OS (p = 0.0016), grade ≥ 2 increased gamma-glutamyltransferase, liver function test abnormalities, pain, and skin/nail reactions (p < 0.05). Low albumin, bulky disease, and mild/moderate hepatic impairment had a significant negative effect on OS (p < 0.01). Modeling supports the recommended loncastuximab tesirine dosing schedule. Although reduced exposure and efficacy were predicted for specific covariates (e.g., low albumin, mild/moderate hepatic impairment), dose increases are not recommended. Trial registration: NCT02669017 and NCT03589469.
Graphical Abstract





Similar content being viewed by others
References
Sapkota S, Shaikh H. Non-Hodgkin lymphoma. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 [Updated 2020 Dec 14]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559328/.
Al-Hamadani M, Habermann TM, Cerhan JR, Macon WR, Maurer MJ, Go RS. Non-Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: a longitudinal analysis of the National Cancer Data Base from 1998 to 2011. Am J Hematol. 2015;90(9):790–5. https://doi.org/10.1002/ajh.24086.
Dreyling M, Ghielmini M, Rule S, Salles G, Vitolo U, Ladetto M. Newly diagnosed and relapsed follicular lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(Suppl 5):v83–90. https://doi.org/10.1093/annonc/mdw400.
Dreyling M, Campo E, Hermine O, Jerkeman M, Le Gouill S, Rule S, et al. Newly diagnosed and relapsed mantle cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(Suppl 4):iv62–71. https://doi.org/10.1093/annonc/mdx223.
Tilly H, Gomes da Silva M, Vitolo U, Jack A, Meignan M, Lopez-Guillermo A, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v116-25. https://doi.org/10.1093/annonc/mdv304.
Chao MP. Treatment challenges in the management of relapsed or refractory non-Hodgkin’s lymphoma - novel and emerging therapies. Cancer Manag Res. 2013;5:251–69. https://doi.org/10.2147/cmar.s34273.
Casulo C, Barr PM. How I treat early-relapsing follicular lymphoma. Blood. 2019;133(14):1540–7. https://doi.org/10.1182/blood-2018-08-822148.
Casulo C, Byrtek M, Dawson KL, Zhou X, Farber CM, Flowers CR, et al. Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the National LymphoCare study. J Clin Oncol. 2015;33(23):2516–22. https://doi.org/10.1200/jco.2014.59.7534.
Sehn LH, Gascoyne RD. Diffuse large B-cell lymphoma: optimizing outcome in the context of clinical and biologic heterogeneity. Blood. 2015;125(1):22–32. https://doi.org/10.1182/blood-2014-05-577189.
Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116(12):2040–5. https://doi.org/10.1182/blood-2010-03-276246.
Sehn LH, Herrera AF, Flowers CR, Kamdar MK, McMillan A, Hertzberg M, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2020;38(2):155–65. https://doi.org/10.1200/jco.19.00172.
Kalakonda N, Maerevoet M, Cavallo F, Follows G, Goy A, Vermaat JSP, et al. Selinexor in patients with relapsed or refractory diffuse large B-cell lymphoma (SADAL): a single-arm, multinational, multicentre, open-label, phase 2 trial. Lancet Haematol. 2020;7(7):e511–22. https://doi.org/10.1016/s2352-3026(20)30120-4.
Salles G, Duell J, González Barca E, Tournilhac O, Jurczak W, Liberati AM, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21(7):978–88. https://doi.org/10.1016/s1470-2045(20)30225-4.
Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56. https://doi.org/10.1056/NEJMoa1804980.
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–44. https://doi.org/10.1056/NEJMoa1707447.
Gisselbrecht C, Van Den Neste E. How I manage patients with relapsed/refractory diffuse large B cell lymphoma. Br J Haematol. 2018;182(5):633–43. https://doi.org/10.1111/bjh.15412.
Wang K, Wei G, Liu D. CD19: a biomarker for B cell development, lymphoma diagnosis and therapy. Exp Hematol Oncol. 2012;1(1):36. https://doi.org/10.1186/2162-3619-1-36.
Zammarchi F, Corbett S, Adams L, Tyrer PC, Kiakos K, Janghra N, et al. ADCT-402, a PBD dimer-containing antibody drug conjugate targeting CD19-expressing malignancies. Blood. 2018;131(10):1094–105. https://doi.org/10.1182/blood-2017-10-813493.
Hamadani M, Radford J, Carlo-Stella C, Caimi PF, Reid E, O’Connor OA, et al. Final results of a phase 1 study of loncastuximab tesirine in relapsed/refractory B-cell non-Hodgkin lymphoma. Blood. 2021;137(19):2634–45. https://doi.org/10.1182/blood.2020007512.
Kahl BS, Hamadani M, Radford J, Carlo-Stella C, Caimi P, Reid E, et al. A phase I study of ADCT-402 (loncastuximab tesirine), a novel pyrrolobenzodiazepine-based antibody-drug conjugate, in relapsed/refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2019;25(23):6986–94. https://doi.org/10.1158/1078-0432.ccr-19-0711.
Caimi PF, Ai W, Alderuccio JP, Ardeshna KM, Hamadani M, Hess B, et al. Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2021;22(6):790–800. https://doi.org/10.1016/s1470-2045(21)00139-x.
Chow SC. Bioavailability and bioequivalence in drug development. Wiley Interdiscip Rev Comput Stat. 2014;6(4):304–12. https://doi.org/10.1002/wics.1310.
ADC Therapeutics. Loncastuximab tesirine population PK report. ADC Therapeutics Inc.: 2020. Run 235.
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–68. https://doi.org/10.1200/jco.2013.54.8800.
Dirks NL, Meibohm B. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49(10):633–59. https://doi.org/10.2165/11535960-000000000-00000.
Lobo ED, Hansen RJ, Balthasar JP. Antibody pharmacokinetics and pharmacodynamics. J Pharm Sci. 2004;93(11):2645–68. https://doi.org/10.1002/jps.20178.
Ryman JT, Meibohm B. Pharmacokinetics of monoclonal antibodies. CPT Pharmacometrics Syst Pharmacol. 2017;6(9):576–88. https://doi.org/10.1002/psp4.12224.
Tokola S, Kuitunen H, Turpeenniemi-Hujanen T, Kuittinen O. Significance of bulky mass and residual tumor—treated with or without consolidative radiotherapy—to the risk of relapse in DLBCL patients. Cancer Med. 2020;9(6):1966–77. https://doi.org/10.1002/cam4.2798.
Carvalho JR, Verdelho MM. New insights about albumin and liver disease. Ann Hepatol. 2018;17(4):547–60. https://doi.org/10.5604/01.3001.0012.0916.
Acknowledgements
The authors would like to thank the patients who participated in the clinical studies and their families. Editorial assistance was provided by Sarah Meadows and Susan Brackenridge, Ph.D., at Fishawack Communications Ltd, part of Fishawack Health, and revision assistance was provided by CiTRUS Health Group.
Funding
The study was supported by ADC Therapeutics. This analysis, as well as studies NCT02669017 and NCT03589469, was sponsored by ADC Therapeutics SA. Pharmax Research was hired by ADC Therapeutics to perform modeling and simulations for this study. Editorial assistance was funded by ADC Therapeutics.
Author information
Authors and Affiliations
Contributions
B.H, W.T, W.A, A.S, M.S, and J.P.A conducted the analysis and interpretation of data, provision of patient care, and writing-review and editing of the manuscript. S.L, L.L, L.K, D.U, X.Z, and J.B conducted the analysis and interpretation of data, conception and design of study, and writing-review and editing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest
B. Hess has had a consulting/advisory role for ADC Therapeutics, an advisory role for Bristol-Myers Squibb and AstraZeneca, and has served on the speaker’s bureau of AstraZeneca. W. Townsend has had a consulting/advisory role for Celgene, Gilead, and Roche; served on the speaker’s bureau of Celgene, Gilead, and Roche; received honoraria from Celgene, Gilead, Roche; received consulting fees from Celgene, Gilead, and Roche; received travel fees from Roche; and is supported by the National Institute for Health Research University College Hospitals Biomedical Research Centre. W. Ai has served on advisory boards for Acrotech Biopharma, ADC Therapeutics, BeiGene, Kymera Therapeutics, and Nurix Therapeutics, and received research funding from Nurix Therapeutics. A. Stathis has had a consulting role for Bayer, received research funding from AbbVie, ADC Therapeutics, Bayer, MEI Pharma, Merck, Novartis, Pfizer, and Roche, and fees for travel from AbbVie and PharmaMar. M. Solh received research funding from ADC Therapeutics and Partner Therapeutics, and served on advisory boards for Bristol-Myers Squibb, Pfizer, and Seattle Genetics, and on a speaker’s bureau for Amgen and Celgene. J.P. Alderuccio was paid for expert testimony by ADC Therapeutics, OncInfo, and OncLive, and he or his immediate family member has served on advisory boards for Agios Pharmaceuticals, Forma Therapeutics, Foundation Medicine, Inovio Pharmaceuticals, and Puma Biotechnology. D. Ungar and J. Boni are employees of ADC Therapeutics and own stock in the company. X. Zhang is a former employee of ADC Therapeutics. S. Liao, L. Liao, and L. Khouri are employees of Pharmax Research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hess, B., Townsend, W., Ai, W. et al. Efficacy and Safety Exposure–Response Analysis of Loncastuximab Tesirine in Patients with B cell non-Hodgkin Lymphoma. AAPS J 24, 11 (2022). https://doi.org/10.1208/s12248-021-00660-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-021-00660-3