Abstract
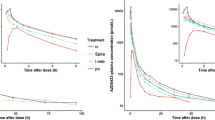
In the context of streamlining generic approval, this study assessed whether pharmacokinetics (PK) could elucidate the pulmonary fate of orally inhaled drug products (OIDPs). Three fluticasone propionate (FP) dry powder inhaler (DPI) formulations (A-4.5, B-3.8, and C-3.7), differing only in type and composition of lactose fines, exhibited median mass aerodynamic diameter (MMAD) of 4.5 μm (A-4.5), 3.8 μm (B-3.8), and 3.7 μm (C-3.7) and varied in dissolution rates (A-4.5 slower than B-3.8 and C-3.7). In vitro total lung dose (TLDin vitro) was determined as the average dose passing through three anatomical mouth-throat (MT) models and yielded dose normalization factors (DNF) for each DPI formulation X (DNFx = TLDin vitro,x/TLDin vitro,A-4.5). The DNF was 1.00 for A-4.5, 1.32 for B-3.8, and 1.21 for C-3.7. Systemic PK after inhalation of 500 μg FP was assessed in a randomized, double-blind, four-way crossover study in 24 healthy volunteers. Peak concentrations (Cmax) of A-4.5 relative to those of B-3.8 or C-3.7 lacked bioequivalence without or with dose normalization. The area under the curve (AUC0–Inf) was bio-IN-equivalent before dose normalization and bioequivalent after dose normalization. Thus, PK could detect differences in pulmonary available dose (AUC0–Inf) and residence time (dose-normalized Cmax). The differences in dose-normalized Cmax could not be explained by differences in in vitro dissolution. This might suggest that Cmax differences may indicate differences in regional lung deposition. Overall this study supports the use of PK studies to provide relevant information on the pulmonary performance characteristics (i.e., available dose, residence time, and regional lung deposition).



Similar content being viewed by others
Change history
22 November 2021
A Correction to this paper has been published: https://doi.org/10.1208/s12248-021-00655-0
Abbreviations
- API:
-
Active pharmaceutical ingredient
- APSD:
-
Aerodynamic particle size distribution
- AUC0–Inf :
-
Area under the plasma concentration time curve from time zero until time infinity
- AUC0–last :
-
Area under the plasma concentration time curve from time zero until the last observed plasma concentration
- AIT:
-
Alberta Idealized Throat
- BE:
-
Bioequivalence
- BSV:
-
Between subject variability
- Cmax :
-
Observed peak concentration in plasma
- CV:
-
Coefficient of variation
- D50:
-
Median particle diameter
- DD:
-
Delivered dose
- DNF:
-
Dose normalization factor
- DPI:
-
Dry powder inhaler
- ECG:
-
Electrocardiogram
- EDTA:
-
Ethylene diamine tetra-acetic acid
- FEV1:
-
Forced expiratory volume in one second
- FP:
-
Fluticasone propionate
- FPD<3 μm:
-
Fine particle dose less than 3 μm
- FPD<5 μm:
-
Fine particle dose less than 5 μm
- GSD:
-
Geometric standard deviation
- HPLC/UV:
-
High performance liquid chromatography with ultraviolet detection
- IND:
-
Investigational new drug
- LC-MS/MS:
-
Liquid chromatography - tandem mass spectrometry
- MMAD:
-
Median mass aerodynamic diameter
- MAT:
-
Mean absorption time
- MDT:
-
Mean dissolution time
- MOC:
-
Micro-orifice collector
- MRT:
-
Mean body residence time
- MT:
-
Mouth-throat models
- NGI:
-
Next generation impactor
- PIFR:
-
Peak inspiratory flow rate
- PK:
-
Pharmacokinetics
- OPC:
-
Oropharyngeal consortium throat
- OIDPs:
-
Orally inhaled drug products
- RH:
-
Relative humidity
- SAP:
-
Statistical analysis plan
- TLDin vitro :
-
In vitro total lung dose
- Tmax :
-
Time to peak concentrations
- VCU:
-
Virginia Commonwealth University
- WSV:
-
Within subject variability
References
Draft guidance on Fluticasone Propionate; Salmeterol Xinofoate. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER); 2013.
Draft guidance on formoterol fumarate; mometasone furoate. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER); 2016.
Draft guidance on beclomethasone dipropionate; mometasone furoate. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER); 2018.
Masoli M, Weatherall M, Holt S, Beasley R. Systematic review of the dose-response relation of inhaled fluticasone propionate. Arch Dis Child. 2004;89(10):902–7.
Barnes PJ, Pedersen S, Busse WW. Efficacy and safety of inhaled corticosteroids. New developments. Am J Respir Crit Care Med. 1998;157(3 Pt 2):S1–53.
Lee SL, Saluja B, Garcia-Arieta A, Santos GM, Li Y, Lu S, et al. Regulatory considerations for approval of generic inhalation drug products in the US, EU, Brazil, China, and India. AAPS J. 2015;17(5):1285–304.
FDA. FYs 2013-2017 Regulatory science report: locally-acting orally inhaled and nasal drug products. https://www.fda.gov/drugs/generic-drugs/fys-2013-2017-regulatory-science-report-locally-acting-orally-inhaled-and-nasal-drug-products. Accessed 9 Mar 2021.
Adams WP, Ahrens RC, Chen ML, Christopher D, Chowdhury BA, Conner DP, et al. Demonstrating bioequivalence of locally acting orally inhaled drug products (OIPs): workshop summary report. J Aerosol Med Pulm Drug Deliv. 2010;23(1):1–29.
Weber B, Hochhaus G. A systematic analysis of the sensitivity of plasma pharmacokinetics to detect differences in the pulmonary performance of inhaled fluticasone propionate products using a model-based simulation approach. AAPS J. 2015;17(4):999–1010.
Boger E, Ewing P, Eriksson UG, Fihn BM, Chappell M, Evans N, et al. A novel in vivo receptor occupancy methodology for the glucocorticoid receptor: toward an improved understanding of lung pharmacokinetic/pharmacodynamic relationships. J Pharmacol Exp Ther. 2015;353(2):279–87.
Harrison TW, Tattersfield AE. Plasma concentrations of fluticasone propionate and budesonide following inhalation from dry powder inhalers by healthy and asthmatic subjects. Thorax. 2003;58(3):258–60.
Olsson B, Bondesson E, Borgström L, Edsbäcker S, Eirefelt S, Ekelund K, et al. Pulmonary drug metabolism, clearance, and absorption. In: Hickey AJ, Smyth HDC, editors. Controlled Pulmonary Drug Delivery. New York: Springer; 2011. p. 21–50.
United States Pharmacopeial Convention. General chapter <905> Uniformity of Dosage Units. United States Pharmacopeia and national formulary (USP 41-NF36). Rockville: United States Pharmacopeial Convention; 2016.
United States Pharmacopeial Convention. General chapter <601> aerosols, nasal sprays, metered dose inhalers, and dry powder inhalers. United States Pharmacopeia and national formulary (USP 41-NF36). United States Pharmacopeial Convention: Rockville; 2016.
Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13(2):123–33.
Wei X, Hindle M, Delvadia RR, Byron PR. In vitro tests for aerosol deposition. V: using realistic testing to estimate variations in aerosol properties at the trachea. J Aerosol Med Pulm Drug Deliv. 2017;30(5):339–48.
Wei X, Hindle M, Kaviratna A, Huynh BK, Delvadia RR, Sandell D, et al. In vitro tests for aerosol deposition. VI: realistic testing with different mouth-throat models and in vitro-in vivo correlations for a dry powder inhaler, metered dose inhaler, and soft mist inhaler. J Aerosol Med Pulm Drug Deliv. 2018;31:358–371.
Xi J, Longest PW. Transport and deposition of micro-aerosols in realistic and simplified models of the oral airway. Ann Biomed Eng. 2007;35(4):560–81.
Burnell PK, Asking L, Borgström L, Nichols SC, Olsson B, Prime D, et al. Studies of the human oropharyngeal airspaces using magnetic resonance imaging IV—the oropharyngeal retention effect for four inhalation delivery systems. J Aerosol Med. 2007;20(3):269–81.
Mitchell J, Mark C, Sizer Y, Russell T, Solomon D. Adapting the Abbreviated Impactor Measurement (AIM) concept to make appropriate inhaler aerosol measurements to compare with clinical data: a scoping study with the “Alberta” Idealized Throat (AIT) inlet. J Aerosol Med Pulmon Drug Deliv. 2012;25(4):188–97.
Delvadia RR, Wei X, Longest PW, Venitz J, Byron PR. In vitro tests for aerosol deposition. IV: simulating variations in human breath profiles for realistic DPI testing. J Aerosol Med Pulm Drug Deliv. 2016;29(2):196–206.
Rohrschneider M, Bhagwat S, Krampe R, Michler V, Breitkreutz J, Hochhaus G. Evaluation of the Transwell system for characterization of dissolution behavior of inhalation drugs: effects of membrane and surfactant. Mol Pharm. 2015;12(8):2618–24.
Bhagwat S, Schilling U, Chen MJ, Wei X, Delvadia R, Absar M, et al. Predicting pulmonary pharmacokinetics from in vitro properties of dry powder inhalers. Pharm Res. 2017;34(12):2541–56.
Bulitta JB, Holford NHG. An introductory guide to non-compartmental analysis. In D'Agostino RB, Sullivan L, Massaro J (ed), Wiley Encyclopedia of Clinical Trials. Hoboken: John Wiley & Sons, Inc; 2008. p 1–28. https://doi.org/10.1002/9780471462422.eoct340.
Krishnaswami S, Möllmann H, Derendorf H, Hochhaus G. A sensitive LC–MS/MS method for the quantification of fluticasone propionate in human plasma. J Pharm Biomed Anal. 2000;22(1):123–9.
Krishnaswami S, Hochhaus G, Derendorf H. An interactive algorithm for the assessment of cumulative cortisol suppression during inhaled corticosteroid therapy. AAPS PharmSci. 2000;2(3):E22.
Kinnunen H, Hebbink G, Peters H, Shur J, Price R. An investigation into the effect of fine lactose particles on the fluidization behaviour and aerosolization performance of carrier-based dry powder inhaler formulations. AAPS PharmSciTech. 2014;15(4):898–909.
Kinnunen H, Shur J, Hebbink G, Muresan AS, Price R. Investigations into the relationship between lactose fluidization properties, device resistance and dry powder inhaler performance. In Dalby, RN, Byron, PR, Peart, J, Suman, JD, Farr, SJ and Young, PM (ed), RDD Conference Paper; 2010. p 791–794.
Kinnunen H, Hebbink G, Peters H, Huck D, Makein L, Price R. Extrinsic lactose fines improve dry powder inhaler formulation performance of a cohesive batch of budesonide via agglomerate formation and consequential co-deposition. Int J Pharm. 2015;478(1):53–9.
Jones MD, Santo JG, Yakub B, Dennison M, Master H, Buckton G. The relationship between drug concentration, mixing time, blending order and ternary dry powder inhalation performance. Int J Pharm. 2010;391(1-2):137–47.
Grasmeijer F, Lexmond AJ, van den Noort M, Hagedoorn P, Hickey AJ, Frijlink HW, et al. New mechanisms to explain the effects of added lactose fines on the dispersion performance of adhesive mixtures for inhalation. PLoS One. 2014;9(1):e87825.
Peng T, Lin S, Niu B, Wang X, Huang Y, Zhang X, et al. Influence of physical properties of carrier on the performance of dry powder inhalers. Acta Pharm Sin B. 2016;6(4):308–18.
Delvadia R, Hindle M, Longest PW, Byron PR. In vitro tests for aerosol deposition II: IVIVCs for different dry powder inhalers in normal adults. J Aerosol Med Pulm Drug Deliv. 2013;26(3):138–44.
Olsson B, Borgstrom L, Lundback H, Svensson M. Validation of a general in vitro approach for prediction of total lung deposition in healthy adults for pharmaceutical inhalation products. J Aerosol Med Pulm Drug Deliv. 2013;26(6):355–69.
Zhang Y, Gilbertson K, Finlay WH. In vivo-in vitro comparison of deposition in three mouth-throat models with Qvar and Turbuhaler inhalers. J Aerosol Med. 2007;20(3):227–35.
Kaviratna A, Tian G, Liu X, Delvadia R, Lee S, Guo C. Evaluation of bio-relevant mouth-throat models for characterization of metered dose inhalers. AAPS PharmSciTech. 2019;20(3):130.
Hochhaus G, Mollmann H, Derendorf H, Gonzalez-Rothi RJ. Pharmacokinetic/pharmacodynamic aspects of aerosol therapy using glucocorticoids as a model. J Clin Pharmacol. 1997;37(10):881–92.
Aswania O, Ritson S, Iqbal SM, Bhatt J, Rigby AS, Everard ML. Intra-subject variability in lung dose in healthy volunteers using five conventional portable inhalers. J Aerosol Med. 2004;17(3):231–8.
Kapitza C, Hompesch M, Scharling B, Heise T. Intrasubject variability of inhaled insulin in type 1 diabetes: a comparison with subcutaneous insulin. Diabetes Technol Ther. 2004;6(4):466–72.
Boger E, Evans N, Chappell M, Lundqvist A, Ewing P, Wigenborg A, et al. Systems pharmacology approach for prediction of pulmonary and systemic pharmacokinetics and receptor occupancy of inhaled drugs. CPT Pharmacometrics Syst Pharmacol. 2016;5(4):201–10.
Weber B, Hochhaus G. A pharmacokinetic simulation tool for inhaled corticosteroids. AAPS J. 2013;15(1):159–71.
Hochhaus G. Using PBPK to link systemic PK to local delivery in the lung. American Society of Clinical Pharmacology and Therapeutics Annual Meeting. Washington: D.C; 2019.
Labiris NR, Dolovich MB. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56(6):588–99.
Funding
Funding for this work was made possible, in part, by the US Food and Drug Administration through contracts HHSF223201110117A and HHSF223201610099C and grants 1U01FD004950 and 1U01FD005231.
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR001427.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclaimer
Views expressed in this article are from the authors and do not necessarily reflect the official policies of the Department of Health and Human Services, the National Institutes of Health, and the FDA, nor does any mention of trade names, commercial practices, or organization imply endorsement by the US Government.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
"The original online version of this article was revised to add article Simon M. Berger at affiliation Department of Pharmaceutics, College of Pharmacy, University of Florida, 1345 Center Drive, Gainesville, Florida, 32610, USA" plus the same explanatory text of the problem as in the erratum/correction article.
Rights and permissions
About this article
Cite this article
Hochhaus, G., Chen, MJ., Kurumaddali, A. et al. Can Pharmacokinetic Studies Assess the Pulmonary Fate of Dry Powder Inhaler Formulations of Fluticasone Propionate?. AAPS J 23, 48 (2021). https://doi.org/10.1208/s12248-021-00569-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-021-00569-x