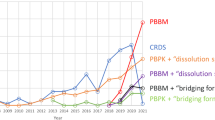
Abstract
Physiologically based pharmacokinetic (PBPK) absorption modeling and simulation is increasingly used as a tool in drug product development, not only in support of clinical pharmacology applications (e.g., drug-drug interaction, dose selection) but also from quality perspective, enhancing drug product understanding. This report provides a summary of the status and the application of PBPK absorption modeling and simulation in new drug application (NDA) submissions to the U.S. Food and Drug Administration to support drug product quality (e.g., clinically relevant dissolution specifications, active pharmaceutical ingredient (API) particle size distribution specifications). During the 10 years from 2008 to 2018, a total of 24 NDA submissions included the use of PBPK absorption modeling and simulations for biopharmaceutics-related assessment. In these submissions, PBPK absorption modeling and simulation served as an impactful tool in establishing the relationship of critical quality attributes (CQAs) including formulation variables, specifically in vitro dissolution, to the in vivo performance. This article also summarizes common practices in PBPK approaches and proposes future directions for the use of PBPK absorption modeling and simulation in drug product quality assessment.
Graphical abstract





Similar content being viewed by others
References
Yu LX, Amidon GL. A compartmental absorption and transit model for estimating oral drug absorption. Int J Pharm. 1999;186(2):119–25.
Zhang X, Lionberger RA, Davit BM, Yu LX. Utility of physiologically based absorption modeling in implementing Quality by Design in drug development. AAPS J. 2011;13(1):59–71.
Willmann S, Schmitt W, Keldenich J, Lippert J, Dressman JB. A physiological model for the estimation of the fraction dose absorbed in humans. Journal of medicinal chemistry. 2004;47(16):4022–31.
Zhang X, Lionberger RA. Modeling and simulation of biopharmaceutical performance. Clin Pharmacol Ther. 2014;95(5):480–2.
Zhao P, Zhang L, Grillo JA, Liu Q, Bullock JM, Moon YJ, et al. Applications of physiologically based pharmacokinetic (PBPK) modeling and simulation during regulatory review. Clin Pharmacol Ther. 2011;89(2):259–67.
Miao L, Mousa YM, Zhao L, Raines K, Seo P, Wu F. Using a physiologically based pharmacokinetic absorption model to establish dissolution bioequivalence safe space for oseltamivir in adult and pediatric populations. AAPS J. 2020;22(5):107. https://doi.org/10.1208/s12248-020-00493-6.
Kesisoglou F, Chung J, van Asperen J, Heimbach T. Physiologically based absorption modeling to impact biopharmaceutics and formulation strategies in drug development-industry case studies. J Pharm Sci. 2016;105:2723–34.
Jones HM, Chen Y, Gibson C, Heimbach T, Parrott N, Peters SA, et al. Physiologically based pharmacokinetic modeling in drug discovery and development: a pharmaceutical industry perspective. Clin Pharmacol Ther. 2015;97(3):247–62.
Heimbach T, Suarez-Sharp S, Kakhi M, Holmstock N, Olivares-Morales A, Pepin X, et al. Dissolution and translational modeling strategies toward establishing an in vitro-in vivo link-a workshop summary report. AAPS J. 2019;21(2):29. https://doi.org/10.1208/s12248-019-0298-x.
U.S. Food and Drug Administration. Guidance for industry: physiologically based pharmacokinetic analyses--format and content 2018; Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM531207.pdf.
U.S. Food and Drug Administration. Draft guidance for industry: the use of physiologically based pharmacokinetic analyses—biopharmaceutics applications for oral drug product development, manufacturing changes, and controls 2020; Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/use-physiologically-based-pharmacokinetic-analyses-biopharmaceutics-applications-oral-drug-product.
European Medicines Agency. Guideline on the reporting of physiologically based pharmacokinetic (PBPK) modeling and simulation. 2018; Available from: https://www.ema.europa.eu/documents/scientific-guideline/guideline-reporting-physiologically-based-pharmacokinetic-pbpk-modelling-simulation_en.pdf.
Wagner C, Pan Y, Hsu V, Grillo JA, Zhang L, Reynolds KS, et al. Predicting the effect of cytochrome P450 inhibitors on substrate drugs: analysis of physiologically based pharmacokinetic modeling submissions to the US Food and Drug Administration. Clin Pharmacokinet. 2015;54(1):117–27.
Wagner C, Pan Y, Hsu V, Sinha V, Zhao P. Predicting the Effect of CYP3A Inducers on the pharmacokinetics of substrate drugs using physiologically based pharmacokinetic (PBPK) modeling: an analysis of PBPK submissions to the US FDA. Clin Pharmacokinet. 2015;54:117–27.
Wu F, Gaohua L, Zhao P, Jamei M, Huang SM, Bashaw ED, et al. Predicting nonlinear pharmacokinetics of omeprazole enantiomers and racemic drug using physiologically based pharmacokinetic modeling and simulation: application to predict drug/genetic interactions. Pharmaceutical research. 2014;31(8):1919–29.
Huang SM, Abernethy DR, Wang Y, Zhao P, Zineh I. The utility of modeling and simulation in drug development and regulatory review. J Pharm Sci. 2013;102(9):2912–23.
Wagner C, Zhao P, Pan Y, Hsu V, Grillo J, Huang SM, et al. Application of Physiologically Based Pharmacokinetic (PBPK) Modeling to support dose selection: report of an FDA Public Workshop on PBPK. CPT Pharmacometrics Syst Pharmacol. 2015;4(4):226–30.
Yu LX, Akseli I, Allen B, Amidon G, Bizjak TG, Boam A, et al. Advancing product quality: a summary of the second FDA/PQRI conference. AAPS J. 2016;18(2):528–43.
Abend A, Heimbach T, Cohen M, Kesisoglou F, Pepin X, Suarez-Sharp S. Dissolution and translational modeling strategies enabling patient-centric drug product development: the M-CERSI Workshop Summary Report. AAPS J. 2018;20(3):60. https://doi.org/10.1208/s12248-018-0213-x.
U.S. Food and Drug Administration. Guidance for Industry: Extended Release Oral Dosage Forms: Development, Evaluation, and Application of In Vitro/In Vivo Correlations. 1997.
Kesisoglou F, Xia B, Agrawal NG. Comparison of deconvolution-based and absorption modeling IVIVC for extended release formulations of a BCS III drug development candidate. AAPS J. 2015;17(6):1492–500.
Mistry B, Patel N, Jamei M, Rostami-Hodjegan A, Martinez MN. Examining the use of a mechanistic model to generate an in vivo/in vitro correlation: journey through a thought process. AAPS J. 2016;18:1144–58.
Mistry B, Patel N, Jamei M, Rostami-Hodjegan A, Martinez MN. Physiologically based in vitro/in vivo correlation (IVIVC) at a population level—-deconvoluting in vivo dissolution and identifying sources of variability (part I) Annual meeting of American Association of Pharmaceutical Science2014.
O'Shea JP, Faisal W, Ruane-O'Hora T, Devine KJ, Kostewicz ES, O'Driscoll CM, et al. Lipidic dispersion to reduce food dependent oral bioavailability of fenofibrate: in vitro, in vivo and in silico assessments. Eur J Pharm Biopharm. 2015;96:207–16.
Suarez-Sharp S, Li M, Duan J, Shah H, Seo P. Regulatory experience with in vivo in vitro correlations (IVIVC) in new drug applications. AAPS J. 2016;18(6):1379–90. https://doi.org/10.1208/s12248-016-9966-2.
Lu AT, Frisella ME, Johnson KC. Dissolution modeling: factors affecting the dissolution rates of polydisperse powders. Pharmaceutical research. 1993;10(9):1308–14.
Wang J, Flanagan DR. General solution for diffusion-controlled dissolution of spherical particles. 1. Theory. J Pharm Sci. 1999;88(7):731–8.
Takano R, Sugano K, Higashida A, Hayashi Y, Machida M, Aso Y, et al. Oral absorption of poorly water-soluble drugs: computer simulation of fraction absorbed in humans from a miniscale dissolution test. Pharmaceutical research. 2006;23(6):1144–56.
Li M, Zhao P, Pan Y, Wagner C. Predictive performance of physiologically based pharmacokinetic models for the effect of food on oral drug absorption: current status. CPT Pharmacometrics Syst Pharmacol. 2018;7(2):82–9. https://doi.org/10.1002/psp4.12260.
Dong Z, Li J, Wu F, Zhao P, Lee SC, Zhang L, et al. Application of physiologically-based pharmacokinetic modeling to predict gastric pH-dependent drug-drug interactions for weak base drugs. CPT Pharmacometrics Syst Pharmacol. 2020;9(8):456–65. https://doi.org/10.1002/psp4.12541.
Xia B, Heimbach T, Lin TH, Li S, Zhang H, Sheng J, et al. Utility of physiologically based modeling and preclinical in vitro/in vivo data to mitigate positive food effect in a BCS class 2 compound. AAPS PharmSciTech. 2013;14(3):1255–66.
Parrott N, Stillhart C, Lindenberg M, Wagner B, Kowalski K, Guerini E, et al. Physiologically based absorption modelling to explore the impact of food and gastric pH changes on the pharmacokinetics of entrectinib. AAPS J. 2020;22(4):78. https://doi.org/10.1208/s12248-020-00463-y.
Riedmaier AE, DeMent K, Huckle J, Bransford P, Stillhart C, Lloyd R, et al. Use of physiologically based pharmacokinetic (PBPK) modeling for predicting drug-food interactions: an industry perspective. AAPS J. 2020;22(6):123. https://doi.org/10.1208/s12248-020-00508-2.
Tistaert C, Heimbach T, Xia B, Parrott N, Samant TS, Kesisoglou F. Food effect projections via physiologically based pharmacokinetic modeling: predictive case studies. J Pharm Sci. 2019;108(1):592–602. https://doi.org/10.1016/j.xphs.2018.05.024.
Zhang L, Wu F, Lee SC, Zhao H, Zhang L. pH-dependent drug-drug interactions for weak base drugs: potential implications for new drug development. Clin Pharmacol Ther. 2014;96(2):266–77.
Mitra A, Parrott N, Miller N, Lloyd R, Tistaert C, Heimbach T, et al. Prediction of pH-dependent drug-drug interactions for basic drugs using physiologically based biopharmaceutics modeling: industry case studies. J Pharm Sci. 2020;109(3):1380–94. https://doi.org/10.1016/j.xphs.2019.11.017.
U.S. Food and Drug Administration. Draft guidance for industry: evaluation of gastric pH-dependent drug interactions with acid-reducing agents: study design, data analysis, and clinical implications guidance for industry. 2020; Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/evaluation-gastric-ph-dependent-drug-interactions-acid-reducing-agents-study-design-data-analysis.
Zhao P, Rowland M, Huang SM. Best practice in the use of physiologically based pharmacokinetic modeling and simulation to address clinical pharmacology regulatory questions. Clin Pharmacol Ther. 2012;92(1):17–20.
Parrott N, Suarez-Sharp S, Kesisoglou F, Pathak SM, Good D, Wagner C, et al. Best practices in the development and validation of physiologically based biopharmaceutics modeling. a workshop summary report. J Pharm Sci. 2020. doi: https://doi.org/10.1016/j.xphs.2020.09.058
Pepin XJH, Dressman J, Parrott N, Delvadia P, Mitra A, Zhang X, et al. In vitro biopredictive methods: a workshop summary report. J Pharm Sci. 2020;110:567–83. https://doi.org/10.1016/j.xphs.2020.09.021.
Mitra A, Suarez-Sharp S, Pepin XJH, Flanagan T, Zhao Y, Kotzagiorgis E, et al. Applications of physiologically based biopharmaceutics modeling (PBBM) to support drug product quality: a workshop summary report. J Pharm Sci. 2020;110:594–609. https://doi.org/10.1016/j.xphs.2020.10.059.
Galia E, Nicolaides E, Horter D, Lobenberg R, Reppas C, Dressman JB. Evaluation of various dissolution media for predicting in vivo performance of class I and II drugs. Pharmaceutical research. 1998;15(5):698–705.
Klein S. The use of biorelevant dissolution media to forecast the in vivo performance of a drug. AAPS J. 2010;12(3):397–406. https://doi.org/10.1208/s12248-010-9203-3.
Nicolaides E, Symillides M, Dressman JB, Reppas C. Biorelevant dissolution testing to predict the plasma profile of lipophilic drugs after oral administration. Pharmaceutical research. 2001;18(3):380–8.
Danielak D, Milanowski B, Wentowski K, Nogowska M, Katny M, Rogowski P, et al. Physiologically Based Dissolution Testing in a Drug Development Process-a Case Study of a successful application in a bioequivalence study of trazodone ER formulations under fed conditions. AAPS PharmSciTech. 2020;21(5):161. https://doi.org/10.1208/s12249-020-01662-8.
Otsuka K, Shono Y, Dressman J. Coupling biorelevant dissolution methods with physiologically based pharmacokinetic modelling to forecast in-vivo performance of solid oral dosage forms. J Pharm Pharmacol. 2013;65(7):937–52. https://doi.org/10.1111/jphp.12059.
Frank KJ, Locher K, Zecevic DE, Fleth J, Wagner KG. In vivo predictive mini-scale dissolution for weak bases: advantages of pH-shift in combination with an absorptive compartment. Eur J Pharm Sci. 2014;61:32–9. https://doi.org/10.1016/j.ejps.2013.12.015.
Heigoldt U, Sommer F, Daniels R, Wagner KG. Predicting in vivo absorption behavior of oral modified release dosage forms containing pH-dependent poorly soluble drugs using a novel pH-adjusted biphasic in vitro dissolution test. Eur J Pharm Biopharm. 2010;76(1):105–11. https://doi.org/10.1016/j.ejpb.2010.05.006.
Lin H, Wang M, Zong Y, Wu F, Raines K, Seo P, et al.Multi-dimensional sensitivity analysis reveals change of sensitivity of oral absorption toward API particle size. Annual Conference of American Association of Pharmaceutical Scientists2016.
Hermans A, Abend AM, Kesisoglou F, Flanagan T, Cohen MJ, Diaz DA, et al. Approaches for establishing clinically relevant dissolution specifications for immediate release solid oral dosage forms. AAPS J. 2017;19(6):1537–49. https://doi.org/10.1208/s12248-017-0117-1.
FDA Public Workshop: Oral absorption modeling and simulation for formulation development and bioequivalence evaluation workshop. 2016 [Accessed on March 22, 2017]; Available from: https://www.fda.gov/Drugs/NewsEvents/ucm488178.htm.
FDA Pharmaceutical Science and Clinical Pharmacology Advisory Committee Meeting 2017; Available from: https://www.fda.gov/AdvisoryCommittees/Calendar/ucm535513.htm.
Pepin XJH, Parrott N, Dressman J, Delvadia P, Mitra A, Zhang X, et al. Current state and future expectations of translational modeling strategies to support drug product development, manufacturing changes and controls: a workshop summary report. J Pharm Sci. 2020;110:555–66. https://doi.org/10.1016/j.xphs.2020.04.021.
Disclaimer
Dr. Ping Zhao participated this work while he was an employee at US FDA. The findings and conclusions contained within are those of the authors and do not necessarily reflect the official positions or policies of the Bill & Melinda Gates Foundation.
Funding
The project is partially supported by FDA MCMi funded Oak Ridge Institute for Science and Education (ORISE) Fellowships. The authors thank Drs Da Xu and Huong Moldthan on collecting some parts of the PBPK data included in regulatory submissions. Drs Da Xu and Huong Moldthan were supported by an appointment to the Research Participation Program at CDER, administered by ORISE through an interagency agreement between the US Department of Energy and the FDA.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, F., Shah, H., Li, M. et al. Biopharmaceutics Applications of Physiologically Based Pharmacokinetic Absorption Modeling and Simulation in Regulatory Submissions to the U.S. Food and Drug Administration for New Drugs. AAPS J 23, 31 (2021). https://doi.org/10.1208/s12248-021-00564-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-021-00564-2