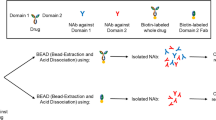
Abstract
The administration of biotherapeutics has the potential to induce potent immune responses. Among these responses, the production of anti-drug antibodies (ADA), including a subset of ADA referred to as neutralizing antibodies (NAb), is of heightened concern. Aside from their capacity to alter the pharmacological profile of a given biotherapeutic, NAb can also pose significant safety risks, especially in instances where an endogenous counterpart to the drug exists. As such, the inclusion of an assay to detect NAb in clinical samples is critical to the effectiveness of a tiered approach to immunogenicity assessment. PF-06730512 is a biotherapeutic protein being developed for the treatment of primary Focal Segmental Glomerulosclerosis (FSGS). To support the immunogenicity assessment of PF-06730512, a cell-based assay was developed for the detection of NAb in FSGS serum samples. Herein, we describe the development of the assay with a focus on the challenges faced, including drug and blood collection tube interferences in NAb detection. The outcome of our efforts was a robust assay capable of detecting 1 μg/mL of a NAb positive control in the presence of clinically relevant drug concentrations up to 30 μg/mL.










Similar content being viewed by others
References
Rosenberg AZ, Kopp JB. Focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2017;12(3):502–17.
Gupta S, Devanarayan V, Finco D, Gunn GR 3rd, Kirshner S, Richards S, et al. Recommendations for the validation of cell-based assays used for the detection of neutralizing antibody immune responses elicited against biological therapeutics. J Pharm Biomed Anal. 2011;55(5):878–88.
Shankar G, Devanarayan V, Amaravadi L, Barrett YC, Bowsher R, Finco-Kent D, et al. Recommendations for the validation of immunoassays used for detection of host antibodies against biotechnology products. J Pharm Biomed Anal. 2008;48(5):1267–81.
Zhang DW, Luo RH, Xu L, Yang LM, Xu XS, Bedwell GJ, et al. A HTRF based competitive binding assay for screening specific inhibitors of HIV-1 capsid assembly targeting the C-terminal domain of capsid. Antivir Res. 2019;169:104544.
Butterfield AM, Chain JS, Ackermann BL, Konrad RJ. Comparison of assay formats for drug-tolerant immunogenicity testing. Bioanalysis. 2010;2(12):1961–9.
Kang YE, Choung S, Lee JH, Kim HJ, Ku BJ. The role of circulating Slit2, the one of the newly batokines, in human diabetes mellitus. Endocrinol Metab (Seoul). 2017;32(3):383–8.
Gouty D, Cai CC, Cai XY, Kasinath A, Kumar V, Alvandkouhi S, et al. Recommendations for the development and validation of neutralizing antibody assays in support of biosimilar assessment. AAPS J. 2017;20(1):25.
Wu B, Chung S, Jiang XR, McNally J, Pedras-Vasconcelos J, Pillutla R, et al. Strategies to determine assay format for the assessment of neutralizing antibody responses to biotherapeutics. AAPS J. 2016;18(6):1335–50.
Xu W, Cummings J, Sank M, Juhel M, Li X, Gleason C, et al. Development and validation of a functional cell-based neutralizing antibody assay for ipilimumab. Bioanalysis. 2018;10(16):1273–87.
Cheung R, de Hart GW, Jesaitis L, Zoog SJ, Melton AC. Detection of antibodies that neutralize the cellular uptake of enzyme replacement therapies with a cell-based assay. J Vis Exp. 2018;(139).
Lallemand C, Kavrochorianou N, Steenholdt C, Bendtzen K, Ainsworth MA, Meritet JF, et al. Reporter gene assay for the quantification of the activity and neutralizing antibody response to TNFalpha antagonists. J Immunol Methods. 2011;373(1–2):229–39.
Bowen RA, Remaley AT. Interferences from blood collection tube components on clinical chemistry assays. Biochem Med (Zagreb). 2014;24(1):31–44.
Acknowledgments
We thank current and former Pfizer colleagues who supported this work: Teresa Caiazzo and Glen Miller (BMD Reagent Group) generated biotinylated PF-06730512; Minlei Zhang (BMD RegBA Group) assisted in method qualification; Wendy Siebert (Global Product Development) facilitated procurement of FSGS serum samples from the NIDDK; Allan McPhee (Clinical Assays Group) supported method implementation and validation at CRO; Dr. Chay Lim and Dr. Susan Hurst provided thoughtful clinical context; Dr. Alison Joyce provided critical review of the manuscript; Dr. Jeffrey Kopp (NIDDK) provided a subset of the FSGS serum samples.
Funding
This work was exclusively funded by Pfizer, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study was funded by Pfizer. All authors listed are employees of Pfizer. Part of the work in this manuscript was presented at the 2019 AAPS D360 conference as a poster.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Luong, M., Wang, Y., Berasi, S.P. et al. Development of a Cell-Based Assay for the Detection of Neutralizing Antibodies to PF-06730512 Using Homogenous Time-Resolved Fluorescence. AAPS J 22, 56 (2020). https://doi.org/10.1208/s12248-020-0431-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-020-0431-x