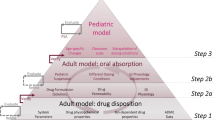
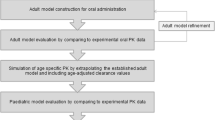
Abstract
Extending licensed drug use to the pediatric population has become an essential part of the drug development process. Nonetheless, ethical concerns limit clinical testing in pediatric populations and data collected from oral bioavailability and food effect studies in adults are often extrapolated to the target pediatric (sub)populations. However, based on published information, food effects on drug absorption in infants may not be adequately evaluated by data collected in adults. In the present study, a physiologically based pharmacokinetic (PBPK) approach for modeling paracetamol suspension data collected in adults was proposed with the ultimate aim to investigate whether extrapolation to infants is substantially affected by the dosing conditions applied to adults. The development of the PBPK model for adults was performed using GastroPlus™ V9.7, and after scaling to infants considering physiological, anatomical, and drug clearance changes, extrapolation of the different dosing conditions was performed by applying dosing conditions dependent on changes on the paracetamol gastric emptying process. Successful simulations of previously observed plasma concentration levels in infants were achieved when extrapolating from fasted and infant formula–fed conditions data. Data collected following the reference meal appeared less useful for simulating paracetamol suspension performance in infants. The proposed methodology deserves further evaluation using high-quality clinical data both in adults and in infants.









Similar content being viewed by others
References
United States Congress 107th. Best pharmaceuticals for children act [Internet]. Vols. 107–109. 2002. p. 1–18. Available from: https://www.congress.gov/107/plaws/publ109/PLAW-107publ109.pdf.
(EU) TEP and TC of the EU. Regulation No 1901/2006. Off J Eur Union [Internet] 2006; Available from: https://ec.europa.eu/health//sites/health/files/files/eudralex/vol-1/reg_2006_1901/reg_2006_1901_en.pdf.
Guimarães M, Statelova M, Holm R, Reppas C, Symilllides M, Vertzoni M, et al. Biopharmaceutical considerations in paediatrics with a view to the evaluation of orally administered drug products—a PEARRL review. J Pharm Pharmacol. 2019;71(4):603–42.
Batchelor H, Kaukonen AM, Klein S, Davit B, Ju R, Ternik R, et al. Food effects in paediatric medicines development for products co-administered with food. Int J Pharm. 2018 Feb;536(2):530–5.
Elder DP, Holm R, Kuentz M. Medicines for pediatric patients—biopharmaceutical, developmental, and regulatory considerations. J Pharm Sci. 2017;106(4):950–60.
Kohlmann P, Stillhart C, Kuentz M, Parrott N. Investigating oral absorption of carbamazepine in pediatric populations. AAPS J. 2017;19(6):1864–77.
Johnson TN, Bonner JJ, Tucker GT, Turner DB, Jamei M. Development and application of a physiologically-based model of paediatric oral drug absorption. Eur J Pharm Sci. 2018;115:57–67.
Verscheijden LFM, Koenderink JB, Johnson TN, De Wildt SN, Russel FGM. Physiologically-based pharmacokinetic models for children: starting to reach maturation ? Pharmacol Ther. 2020;10.
Statelova M, Goumas K, Fotaki N, Holm R, Symillides M, Reppas C, et al. On the design of food effect studies in adults for extrapolating Oral drug absorption data to infants : an exploratory study highlighting the importance of infant food. AAPS J. 2020;22(6):1–11.
Maharaj AR, Edginton AN. Physiologically based pharmacokinetic modeling and simulation in pediatric drug development. CPT Pharmacometrics Syst Pharmacol. 2014;3(November):e150.
Food and Drug Administration (FDA). Assessing the effects of food on drugs in INDs and NDAs-clinical pharmacology considerations guidance for industry [Internet]. 2019. Available from: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm.
Batchelor H. Influence of food on paediatric gastrointestinal drug absorption following oral administration: a review. Children. 2015;2(2):244–71.
European Medicines Agency (EMA). Guideline on the investigation of drug interactions. Guid Doc [Internet]. 2012;44(June):1–59. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/07/WC500129606.pdf.
Food and Drug Administration (FDA). Guidance for industry food-effect bioavailability and fed bioequivalance studies. 2002;(December):1–12. Available from: https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm070241.pdf.
Atkinson HC, Stanescu I, Frampton C, Salem II, Beasley CPH, Robson R. Pharmacokinetics and bioavailability of a fixed-dose combination of ibuprofen and paracetamol after intravenous and oral administration. Clin Drug Investig. 2015;35(10):625–32.
Clements J, Critchley J, Prescott L. The role of sulphate conjugation in the metabolism and disposition of oral and intravenous paracetamol in man. Br J Clin Pharmacol. 1984;18(4):481–5.
De Morais S, Uetrecht J, Wells P. Decreased glucuronidation and increased bioactivation of acetaminophen in Gilbert’s syndrome. Gastroenterology. 1992;102(2):577–86.
Douglas AP, Savage RL, Rawlins MD. Paracetamol (acetaminophen) kinetics in patients with Gilbert’s syndrome. Eur J Clin Pharmacol. 1978;13(3):209–12.
Eandi M, Viano I, Gamalero SR. Absolute bioavailability of paracetamol after oral or rectal administration in healthy volunteers. Drug Res (Stuttg). 1984;34(8):903–7.
Liukas A, Kuusniemi K, Aantaa R, Virolainen P, Niemi M, Neuvonen PJ, et al. Pharmacokinetics of intravenous paracetamol in elderly patients. Clin Pharmacokinet. 2011;50(2):121–9.
Perucca E, Richens A. Paracetamol disposition in normal subjects and in patients treated with antiepileptic drugs. Br J Clin Pharmacol. 1979;7(2):201–6.
Prescott LF, Speirs GC, Critchley JA, Temple RM, Winney RJ. Paracetamol disposition and metabolite kinetics in patients with chronic renal failure. Eur J Clin Pharmacol. 1989;36(3):291–7.
Rawlins MD, Henderson DB, Hijab AR. Pharmacokinetics of paracetamol (acetaminophen) after intravenous and oral administration. Eur J Clin Pharmacol. 1977;11:283–6.
Singla NK, Parulan C, Samson R, Hutchinson J, Bushnell R, Beja EG, et al. Plasma and cerebrospinal fluid pharmacokinetic parameters after single-dose administration of intravenous, oral, or rectal acetaminophen. Pain Pract. 2012;12(7):523–32.
Zuppa A, Hammer G, Barrett J, Kenney B, Kassir N, Moukassi S, et al. Safety and population pharmacokinetic analysis of intravenous acetaminophen in neonates, infants, children, and adolescents with pain or fever. J Pediatr Pharmacol Ther. 2011;16(4):246–61.
Mohammed BS, Engelhardt T, Cameron GA, Cameron L, Hawksworth GM, Hawwa AF, et al. Population pharmacokinetics of single-dose intravenous paracetamol in children. Br J Anaesth. 2012;108(5):823–9.
Hopkins CS, Underhill S, Booker PD. Pharmacokinetics of paracetamol after cardiac surgery. Arch Dis Child. 1990;65(9):971–6.
Walson PD, Halvorsen M, Edge J, Casavant MJ, Kelley MT. Pharmacokinetic comparison of acetaminophen elixir versus suppositories in vaccinated infants (aged 3 to 36 months): a single-dose, open-label, randomized, Parallel-Group design. Clin Ther. 2013;35(2):135–40.
Tsamandouras N, Rostami-Hodjegan A, Aarons L. Combining the “bottom up” and “top down” approaches in pharmacokinetic modelling: fitting PBPK models to observed clinical data. Br J Clin Pharmacol. 2015;79(1):48–55.
Jamei M. Recent advances in development and application of physiologically-based pharmacokinetic (PBPK) models: a transition from academic curiosity to regulatory acceptance. Curr Pharmacol Rep. 2016;2(3):161–9.
Kalantzi L, Reppas C, Dressman JB, Amidon GL, Junginger HE, Midha KK, et al. Biowaiver monographs for immediate release solid oral dosage forms: acetaminophen (Paracetamol). J Pharm Sci. 2006;95(1):4–14.
Jiang X-L, Zhao P, Barrett JS, Lesko LJ, Schmidt S. Application of physiologically based pharmacokinetic modeling to predict acetaminophen metabolism and pharmacokinetics in children. CPT Pharmacometrics Syst Pharmacol. 2013;2(August):e80.
Ladumor MK, Bhatt DK, Gaedigk A, Sharma S, Thakur A, Pearce RE, et al. Ontogeny of hepatic sulfotransferases and prediction of age-dependent fractional contribution of sulfation in acetaminophen metabolism. Drug Metab Dispos. 2019;47(8):818–31.
Yamashita S, Furubayashi T, Kataoka M, Sakane T, Sezaki H, Tokuda H. Optimized conditions for prediction of intestinal drug permeability using Caco-2 cells. Eur J Pharm Sci. 2000;10(3):195–204.
Sun D, Lennernas H, Welage LS, Barnett JL, Landowski CP, Foster D, et al. Comparison of human duodenum and Caco-2 gene expression profiles for 12,000 gene sequences tags and correlation with permeability of 26 drugs. Pharm Res. 2002;19(10):1400–16.
Strougo A, Eissing T, Yassen A, Willmann S, Danhof M, Freijer J. First dose in children: physiological insights into pharmacokinetic scaling approaches and their implications in paediatric drug development. J Pharmacokinet Pharmacodyn. 2012;39(2):195–203.
Prescott L. Kinetics and metabolism of paracetamol and phenacetin. Br J Clin Pharmacol. 1980;10(2 S):291S–8S.
Rodgers T, Rowland M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and Zwitterions. J Pharm Sci. 2005;95(6):1238–57.
Lukacova V, Parrott NJ, Lave T, Fraczkiewicz G, Bolger MB. Role of fraction unbound in plasma in calculation of tissue:plasmapartition coefficients. In: AAPS National meeting, Atlanta, November 15–20. 2008.
Laine JE, Auriola S, Pasanen M, Juvonen RO. Acetaminophen bioactivation by human cytochrome P450 enzymes and animal microsomes. Xenobiotica. 2009;39(1):11–21.
Mutlib AE, Goosen TC, Bauman JN, Williams JA, Kulkarni S, Kostrubsky S. Kinetics of acetaminophen glucuronidation by UDP-glucuronosyltransferases 1A1, 1A6, 1A9 and 2B15. Potential implications in acetaminophen-induced hepatotoxicity. Chem Res Toxicol. 2006;19(5):701–9.
Adjei AA, Gaedigk A, Simon SD, Weinshilboum RM, Leeder JS. Interindividual variability in acetaminophen sulfation by human fetal liver: implications for pharmacogenetic investigations of drug-induced birth defects. Birth Defects Res A Clin Mol Teratol. 2008;82(3):155–65.
SimulationsPlus Inc. GastroPlus Manual.
T’jollyn H, Vermeulen A, Van Bocxlaer J. PBPK and its virtual populations: the impact of physiology on pediatric pharmacokinetic predictions of tramadol. AAPS J. 2019;21(1):1–12.
Villiger A, Stillhart C, Parrott N, Kuentz M. Using physiologically based pharmacokinetic (PBPK) modelling to gain insights into the effect of physiological factors on oral absorption in paediatric populations. AAPS J. 2016;18(4):933–47.
Edginton AN, Willmann S. Physiology-based versus allometric scaling of clearance in children: a comparison. 2006;51368.
Lowenthal DT, Oie S, van Stone JC, Briggs WA, Levy G. Pharmacokinetics of acetaminophen elimination by anephric patients. J Pharmacol Exp Ther. 1976;196(3):570–8.
Terry SI, Gould JC, McManus JPA, Prescott LF. Absorption of penicillin and paracetamol after small intestinal bypass surgery. Eur J Clin Pharmacol. 1982;23(3):245–8.
Boase S, Miners JO. In vitro-in vivo correlations for drugs eliminated by glucuronidation: investigations with the model substrate zidovudine. Br J Clin Pharmacol. 2002;54(5):493–503.
Agoram B, Woltosz WS, Bolger MB. Predicting the impact of physiological and biochemical processes on oral drug bioavailability. Adv Drug Deliv Rev. 2001;50:S41–67.
Kostewicz ES, Aarons L, Bergstrand M, Bolger MB, Galetin A, Hatley O, et al. PBPK models for the prediction of in vivo performance of oral dosage forms. Eur J Pharm Sci. 2014;57(1):300–21.
Willems M, Quartero AO, Numans ME. How useful is paracetamol absorption as a marker of gastric emptying? A systematic literature study. Dig Dis Sci. 2001;46(10):2256–62.
Johnson TN, Rostami-hodjegan A, Tucker GT. Prediction of the clearance of eleven drugs and associated variability in neonates. Infants Child. 2006;45(9):931–56.
Badée J, Qiu N, Collier AC, Takahashi RH, Forrest WF, Parrott N, et al. Characterization of the ontogeny of hepatic UDP-glucuronosyltransferase enzymes based on glucuronidation activity measured in human liver microsomes. J Clin Pharmacol. 2019;59(S1):S42–55.
Bonner JJ, Vajjah P, Abduljalil K, Jamei M, Rostami-Hodjegan A, Tucker GT, et al. Does age affect gastric emptying time? A model-based meta-analysis of data from premature neonates through to adults. Biopharm Drug Dispos. 2015;36(4):245–57.
Obach RS, Baxter JG, Liston TE, Silber BM, Jones BC, Macintyre F, et al. The prediction of human pharmacokinetic parameters from preclinical and in vitro metabolism data. J Pharmacol Exp Ther. 1997;283(1):46 LP–58.
Mahmood I, Ahmad T, Mansoor N, Sharib SM. Prediction of clearance in neonates and infants (≤ 3 months of age) for drugs that are Glucuronidated: a comparative study between allometric scaling and physiologically based pharmacokinetic modeling. J Clin Pharmacol. 2017;57(4):476–83.
Lennernäs H. Human in vivo regional intestinal permeability: importance for pharmaceutical drug development. Mol Pharm. 2014;11(1):12–23.
Levitt DG. Quantitation of small intestinal permeability during normal human drug absorption. BMC Pharmacol Toxicol. 2013;14(1):1.
Sanaka M, Kuyama Y, Shimomura Y, Saitoh M, Hattori K. New mathematical model for accurate description of absorption kinetics of paracetamol given orally with a high calorie liquid meal. Int J Clin Pharmacol Ther. 2002;40(11):499–506.
Bermejo M, Hens B, Dickens J, Mudie D, Paix P. A mechanistic physiologically-based biopharmaceutics modeling (PBBM) approach to assess the in vivo performance of an orally administered drug product : from IVIVC to IVIVP. Pharmaceutics. 2020;12(1):74.
Van Den Abeele J, Brouwers J, Tack J, Augustijns P. Exploring the link between gastric motility and intragastric drug distribution in man. Eur J Pharm Biopharm. 2017;112:75–84.
Grimm M, Scholz E, Koziolek M, Kuhn JP, Weitschies W. Gastric water emptying under fed state clinical trial conditions is as fast as under fasted conditions gastric water emptying under fed state clinical trial conditions is as fast as under fasted conditions. Mol Pharm. 2017;14(12):4262–71.
Pentafragka C, Vertzoni M, Symillides M, Goumas K, Reppas C. Disposition of two highly permeable drugs in the upper gastrointestinal lumen of healthy adults after a standard high-calorie, high-fat meal. Eur J Pharm Sci. 2020;149(January).
Van Den Abeele J, Rayyan M, Hoffman I, Van de Vijver E, Zhu W, Augustijns P. Gastric fluid composition in a paediatric population: age-dependent changes relevant for gastrointestinal drug disposition. Eur J Pharm Sci. 2018;123:301–11.
Yeung CHT, Fong S, Malik PRV, Edginton AN. Quantifying breast milk intake by term and preterm infants for input into paediatric physiologically based pharmacokinetic models. Matern Child Nutr. 2020;16(2):1–33.
Koziolek M, Alcaro S, Augustijns P, Basit AW, Grimm M, Hens B, et al. The mechanisms of pharmacokinetic food-drug interactions – a perspective from the UNGAP group. Eur J Pharm Sci. 2019;134(March):31–59.
Hauser B, Roelants M, De Schepper J, Veereman G, Caveliers V, Devreker T, et al. Gastric emptying of liquids in children. J Pediatr Gastroenterol Nutr. 2015;62(3):403–8.
Lange A, Funch-Jensen P, Thommesen P, Schiøtz PO. Gastric emptying patterns of a liquid meal in newborn infants measured by epigastric impedance. Neurogastroenterol Motil. 1997;9(2):55–62.
Johnson TN, Bonner JJ, Tucker GT, Turner DB, Jamei M. Development and applications of a physiologically-based model of paediatric oral drug absorption. Eur J Pharm Sci. 2018;115(June 2017):57–67.
Billeaud C, Guillet J, Sandler B. Gastric emptying in infants with or without gastroesophageal reflux according to the type of milk. Eur J Clin Nutr. 1990;44(September 1990):577–83.
Cavell B. Gastric emptying in infants with cycstic fibrosis. Acta Paediatr Scand. 1981;70:635–8.
Staelens S, Van Den Driessche M, Barclay D, Carrié-Faessler AL, Haschke F, Verbeke K, et al. Gastric emptying in healthy newborns fed an intact protein formula, a partially and an extensively hydrolysed formula. Clin Nutr. 2008;27(2):264–8.
Van Den Driessche M, Veereman-Wauters G. Gastric emptying in infants and children. Acta Gastroenterol Belg. 2003;66(4):274–82.
Lee GO, McCormick BJJ, Seidman JC, Kosek MN, Haque R, Olortegui MP, et al. Infant nutritional status, feeding practices, enteropathogen exposure, socioeconomic status, and illness are associated with gut barrier function as assessed by the lactulose mannitol test in the MAL-ED birth cohort. Am J Trop Med Hyg. 2017;97(1):281–90.
Riezzo G, Indrio F, Raimondi F, Montagna O, Salvia G, Massimo B, et al. Maturation of gastric electrical activity, gastric emptying and intestinal permeability in preterm newborns during the first month of life. Ital J Pediatr. 2009 Mar 15;35(1):6.
Corpeleijn WE, van Vliet I, de Gast-Bakker D-AH, van der Schoor SRD, Alles MS, Hoijer M, et al. Effect of enteral IGF-1 supplementation on feeding tolerance, growth, and gut permeability in enterally fed premature neonates. J Pediatr Gastroenterol Nutr. 2008;46(2).
Saleem B, Okogbule-Wonodi AC, Fasano A, Magder LS, Ravel J, Kapoor S, et al. Intestinal barrier maturation in very low birthweight infants: relationship to feeding and antibiotic exposure. J Pediatr. 2017;183:31–36.e1.
Shulman RJ, Schanler RJ, Lau C, Heitkemper M, Ou C-N, Smith EO. Early feeding, antenatal glucocorticoids, and human milk decrease intestinal permeability in preterm infants. Pediatr Res. 1998;44(4):519–23.
Acknowledgments
The authors would like to express their gratitude to SimulationsPlus Inc. for their support and providing access to the latest software version of the PBPK modeling platform GastroPlus™ 9.7.
Funding
This work has received funding from Horizon 2020 Marie Sklodowska-Curie Innovative Training Networks programme under grant agreement no. 674909.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editor: Filippos Kesisoglou
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 3.50 mb)
Rights and permissions
About this article
Cite this article
Statelova, M., Holm, R., Fotaki, N. et al. Successful Extrapolation of Paracetamol Exposure from Adults to Infants After Oral Administration of a Pediatric Aqueous Suspension Is Highly Dependent on the Study Dosing Conditions. AAPS J 22, 126 (2020). https://doi.org/10.1208/s12248-020-00504-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-020-00504-6