Abstract
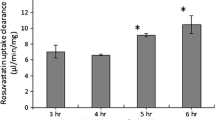
During drug development, in vivo human biliary drug clearances (CL) are usually predicted using human sandwich-cultured hepatocytes (SCH). To do so, SCH are pre-incubated with Ca2+-containing or Ca2+-free buffer to maintain or disrupt canalicular tight junctions (CTJ), respectively. Drug uptake into SCH is then conducted in the presence of Ca2+ (up to 20 min). Under this standard protocol, two key assumptions are made: first, that the CTJ are not reformed during the uptake phase when Ca2+ is repleted, and second, disruption of CTJ by the Ca2+-free buffer does not affect the activity of any of the transporters present in the sinusoidal or canalicular membrane. Here we investigated the validity of these assumptions using rosuvastatin (RSV) and taurocholic acid (TCA) as our model drugs. In human SCH, the disrupted CTJ were “reformed” with just 10-min Ca2+ repletion as reflected in a significant increase in TCA cell accumulation. To avoid CTJ reformation and cell toxicity, the standard SCH protocol was modified by conducting the uptake in the absence of Ca2+ for 10 min. Surprisingly, using this protocol, RSV uptake into SCH, plated hepatocytes, and transporter-expressing cells confirmed that Ca2+ depletion substantially decreased NTCP and not OATP1B1 activity. Collectively, this study provides the first evidence of reformation of CTJ in human SCH with 20-min Ca2+ repletion, whereas Ca2+ depletion, during the uptake phase, leads to a significant reduction in NTCP uptake. Thus, the entire SCH protocol needs to be re-examined and optimized to correctly estimate hepatobiliary CL of drugs including those that are NTCP substrates.







Similar content being viewed by others
Abbreviations
- BCA:
-
Bicinchoninic acid assay
- BCRP:
-
Breast cancer resistance protein
- CL:
-
Clearance
- HBSS:
-
Hank’s balanced salt solution
- LC-MS/MS:
-
Liquid chromatography tandem mass spectrometry
- LDH:
-
Lactate dehydrogenase
- NTCP:
-
Sodium-taurocholate co-transporting polypeptide
- OATP:
-
Organic anion transporting polypeptide
- RSV:
-
Rosuvastatin
- SCH:
-
Sandwich-cultured hepatocytes
- SDS:
-
Sodium dodecyl sulfate
- TCA:
-
Taurocholic acid
References
Ghibellini G, Leslie EM, Brouwer KL. Methods to evaluate biliary excretion of drugs in humans: an updated review. Mol Pharm. 2006;3(3):198–211. https://doi.org/10.1021/mp060011k.
Patilea-Vrana G, Unadkat JD. Transport vs. metabolism: what determines the pharmacokinetics and pharmacodynamics of drugs? Insights from the extended clearance model. Clin Pharmacol Ther. 2016;100(5):413–8. https://doi.org/10.1002/cpt.437.
Hosey CM, Broccatelli F, Benet LZ. Predicting when biliary excretion of parent drug is a major route of elimination in humans. AAPS J. 2014;16(5):1085–96. https://doi.org/10.1208/s12248-014-9636-1.
Swift B, Pfeifer ND, Brouwer KL. Sandwich-cultured hepatocytes: an in vitro model to evaluate hepatobiliary transporter-based drug interactions and hepatotoxicity. Drug Metab Rev. 2010;42(3):446–71. https://doi.org/10.3109/03602530903491881.
Pfeifer ND, Yang K, Brouwer KL. Hepatic basolateral efflux contributes significantly to rosuvastatin disposition I: characterization of basolateral versus biliary clearance using a novel protocol in sandwich-cultured hepatocytes. J Pharmacol Exp Ther. 2013;347(3):727–36. https://doi.org/10.1124/jpet.113.207472.
Liu X, LeCluyse EL, Brouwer KR, Lightfoot RM, Lee JI, Brouwer KL. Use of Ca2+ modulation to evaluate biliary excretion in sandwich-cultured rat hepatocytes. J Pharmacol Exp Ther. 1999;289(3):1592–9.
Mukhopadhyay S, Ananthanarayanan M, Stieger B, Meier PJ, Suchy FJ, Anwer MS. Sodium taurocholate cotransporting polypeptide is a serine, threonine phosphoprotein and is dephosphorylated by cyclic adenosine monophosphate. Hepatology. 1998;28(6):1629–36. https://doi.org/10.1002/hep.510280624.
McTaggart F. Comparative pharmacology of rosuvastatin. Atheroscler Suppl. 2003;4(1):9–14. https://doi.org/10.1016/s1567-5688(03)00004-7.
Ho RH, Tirona RG, Leake BF, Glaeser H, Lee W, Lemke CJ, et al. Drug and bile acid transporters in rosuvastatin hepatic uptake: function, expression, and pharmacogenetics. Gastroenterology. 2006;130(6):1793–806. https://doi.org/10.1053/j.gastro.2006.02.034.
Abe K, Bridges AS, Yue W, Brouwer KL. In vitro biliary clearance of angiotensin II receptor blockers and 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors in sandwich-cultured rat hepatocytes: comparison with in vivo biliary clearance. J Pharmacol Exp Ther. 2008;326(3):983–90. https://doi.org/10.1124/jpet.108.138073.
Kitamura S, Maeda K, Wang Y, Sugiyama Y. Involvement of multiple transporters in the hepatobiliary transport of rosuvastatin. Drug Metab Dispos. 2008;36(10):2014–23. https://doi.org/10.1124/dmd.108.021410.
Huang L, Wang Y, Grimm S. ATP-dependent transport of rosuvastatin in membrane vesicles expressing breast cancer resistance protein. Drug Metab Dispos. 2006;34(5):738–42. https://doi.org/10.1124/dmd.105.007534.
Keskitalo JE, Zolk O, Fromm MF, Kurkinen KJ, Neuvonen PJ, Niemi M. ABCG2 polymorphism markedly affects the pharmacokinetics of atorvastatin and rosuvastatin. Clin Pharmacol Ther. 2009;86(2):197–203. https://doi.org/10.1038/clpt.2009.79.
Jemnitz K, Veres Z, Tugyi R, Vereczkey L. Biliary efflux transporters involved in the clearance of rosuvastatin in sandwich culture of primary rat hepatocytes. Toxicol in Vitro. 2010;24(2):605–10. https://doi.org/10.1016/j.tiv.2009.10.009.
Hagenbuch B, Meier PJ. Sinusoidal (basolateral) bile salt uptake systems of hepatocytes. Semin Liver Dis. 1996;16(2):129–36. https://doi.org/10.1055/s-2007-1007226.
Kumar V, Salphati L, Hop C, Xiao G, Lai Y, Mathias A, et al. A comparison of total and plasma membrane abundance of transporters in suspended, plated, sandwich-cultured human hepatocytes versus human liver tissue using quantitative targeted proteomics and cell surface biotinylation. Drug Metab Dispos. 2019;47(4):350–7. https://doi.org/10.1124/dmd.118.084988.
Abe K, Bridges AS, Brouwer KL. Use of sandwich-cultured human hepatocytes to predict biliary clearance of angiotensin II receptor blockers and HMG-CoA reductase inhibitors. Drug Metab Dispos. 2009;37(3):447–52. https://doi.org/10.1124/dmd.108.023465.
Annaert P, Ye ZW, Stieger B, Augustijns P. Interaction of HIV protease inhibitors with OATP1B1, 1B3, and 2B1. Xenobiotica. 2010;40(3):163–76. https://doi.org/10.3109/00498250903509375.
Kim RB, Leake B, Cvetkovic M, Roden MM, Nadeau J, Walubo A, et al. Modulation by drugs of human hepatic sodium-dependent bile acid transporter (sodium taurocholate cotransporting polypeptide) activity. J Pharmacol Exp Ther. 1999;291(3):1204–9.
Anwer MS, Clayton LM. Role of extracellular Ca2+ in hepatic bile formation and taurocholate transport. Am J Phys. 1985;249(6 Pt 1):G711–8. https://doi.org/10.1152/ajpgi.1985.249.6.G711.
Pan G, Boiselle C, Wang J. Assessment of biliary clearance in early drug discovery using sandwich-cultured hepatocyte model. J Pharm Sci. 2012;101(5):1898–908. https://doi.org/10.1002/jps.23070.
Nakakariya M, Ono M, Amano N, Moriwaki T, Maeda K, Sugiyama Y. In vivo biliary clearance should be predicted by intrinsic biliary clearance in sandwich-cultured hepatocytes. Drug Metab Dispos. 2012;40(3):602–9. https://doi.org/10.1124/dmd.111.042101.
Gonzalez-Mariscal L, Chavez de Ramirez B, Cereijido M. Tight junction formation in cultured epithelial cells (MDCK). J Membr Biol. 1985;86(2):113–25. https://doi.org/10.1007/BF01870778.
Grune S, Engelking LR, Anwer MS. Role of intracellular calcium and protein kinases in the activation of hepatic Na+/taurocholate cotransport by cyclic AMP. J Biol Chem. 1993;268(24):17734–41.
Exton JH. Role of phosphoinositides in the regulation of liver function. Hepatology. 1988;8(1):152–66. https://doi.org/10.1002/hep.1840080129.
Ishida K, Ullah M, Toth B, Juhasz V, Unadkat JD. Successful prediction of in vivo hepatobiliary clearances and hepatic concentrations of rosuvastatin using sandwich-cultured rat hepatocytes, transporter-expressing cell lines, and quantitative proteomics. Drug Metab Dispos. 2018;46(1):66–74. https://doi.org/10.1124/dmd.117.076539.
Kumar V, Yin J, Billington S, Prasad B, Brown CDA, Wang J, et al. The importance of incorporating OCT2 plasma membrane expression and membrane potential in IVIVE of metformin renal secretory clearance. Drug Metab Dispos. 2018;46(10):1441–5. https://doi.org/10.1124/dmd.118.082313.
Acknowledgments
The authors thank Tot Bui Nguyen for her support in human hepatocytes and transporter-expressing cell line culture. We thank BioIVT for providing the human hepatocytes.
Funding
This work was supported by University of Washington Research Affiliate Program on Transporters (UWRAPT) through funding from Gilead Sciences, Amgen, Biogen, Genentech, Merck, Pfizer, and Takeda. VK was supported in part by the Simcyp Grant & Partnership Scheme.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
EK and ZG are employees of SOLVO Biotechnology, Budapest, Hungary. KI is an employee of Gilead Sciences.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 171 kb)
Rights and permissions
About this article
Cite this article
Kumar, V., Li, C.Y., Ishida, K. et al. Pitfalls in Predicting Hepatobiliary Drug Transport Using Human Sandwich-Cultured Hepatocytes. AAPS J 22, 110 (2020). https://doi.org/10.1208/s12248-020-00496-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-020-00496-3