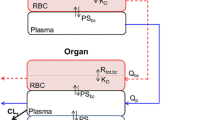
Abstract
Bortezomib is a potent 20S proteasome inhibitor approved for the treatment of multiple myeloma and mantle cell lymphoma. Despite the extensive clinical use of bortezomib, the mechanism of the complex time-dependent pharmacokinetics of bortezomib has not been fully investigated in context of its pharmacodynamics (PD) and drug–drug interaction (DDI) profiles. Here, we aimed to develop a mechanistic physiologically based (PB) PK/PD model to project PK, blood target inhibition and DDI of bortezomib in patients. A minimal PBPK/PD model consisting of six compartments was constructed using a bottom–up approach with pre-clinical data and human physiological parameters. Specifically, the target-mediated drug disposition (TMDD) of bortezomib in red blood cells (RBC), which determines target inhibition in blood, was characterized by incorporating the proteasome binding affinity of bortezomib and the proteasome concentration in RBC. The hepatic clearance and fraction metabolized by different CYP isoforms were estimated from in vitro metabolism and phenotyping experiments. The established model adequately characterized the multi-exponential and time-dependent plasma pharmacokinetics, target binding and blood proteasome inhibition of bortezomib. Further, the model was able to accurately predict the impact of a strong CYP3A inducer (rifampicin) and inhibitor (ketoconazole) on bortezomib exposure. In conclusion, the mechanistic PBPK/PD model successfully described the complex pharmacokinetics, target inhibition and DDIs of bortezomib in patients. This study illustrates the importance of incorporating target biology, drug–target interactions and in vitro clearance parameters into mechanistic PBPK/PD models and the utility of such models for pharmacokinetic, pharmacodynamic and DDI predictions.




Similar content being viewed by others
References
Adams J, Kauffman M. Development of the proteasome inhibitor VELCADE (bortezomib). Cancer Investig. 2004;22(2):304–11.
Adams J. The development of proteasome inhibitors as anticancer drugs. Cancer Cell. 2004;5(5):417–21.
Boccadoro M, Morgan G, Cavenagh J. Preclinical evaluation of the proteasome inhibitor bortezomib in cancer therapy. Cancer Cell Int. 2005;5(1):18.
Reece DE, Sullivan D, Lonial S, Mohrbacher AF, Chatta G, Shustik C, et al. Pharmacokinetic and pharmacodynamic study of two doses of bortezomib in patients with relapsed multiple myeloma. Cancer Chemother Pharmacol. 2011;67(1):57–67.
Quinn DI, Nemunaitis J, Fuloria J, Britten CD, Gabrail N, Yee L, et al. Effect of the cytochrome P450 2C19 inhibitor omeprazole on the pharmacokinetics and safety profile of bortezomib in patients with advanced solid tumours, non-Hodgkin’s lymphoma or multiple myeloma. Clin Pharmacokinet. 2009;48(3):199–209.
Zhang L, Mager DE. Physiologically-based pharmacokinetic modeling of target-mediated drug disposition of bortezomib in mice. J Pharmacokinet Pharmacodyn. 2015;42(5):541–52.
Hemeryck A, Geerts R, Monbaliu J, Hassler S, Verhaeghe T, Diels L, et al. Tissue distribution and depletion kinetics of bortezomib and bortezomib-related radioactivity in male rats after single and repeated intravenous injection of 14 C-bortezomib. Cancer Chemother Pharmacol. 2007;60(6):777–87.
LoRusso PM, Venkatakrishnan K, Ramanathan RK, Sarantopoulos J, Mulkerin D, Shibata SI, et al. Pharmacokinetics and safety of bortezomib in patients with advanced malignancies and varying degrees of liver dysfunction: phase I NCI Organ Dysfunction Working Group Study NCI-6432. Clin Cancer Res. 2012;18(10):2954–63.
Pekol T, Daniels JS, Labutti J, Parsons I, Nix D, Baronas E, et al. Human metabolism of the proteasome inhibitor bortezomib: identification of circulating metabolites. Drug Metab Dispos. 2005;33(6):771–7.
Uttamsingh V, Lu C, Miwa G, Gan LS. Relative contributions of the five major human cytochromes P450, 1A2, 2C9, 2C19, 2D6, and 3A4, to the hepatic metabolism of the proteasome inhibitor bortezomib. Drug Metab Dispos. 2005;33(11):1723–8.
Hellmann A, Rule S, Walewski J, Shpilberg O, Feng H, van de Velde H, et al. Effect of cytochrome P450 3A4 inducers on the pharmacokinetic, pharmacodynamic and safety profiles of bortezomib in patients with multiple myeloma or non-Hodgkin's lymphoma. Clin Pharmacokinet. 2011;50(12):781–91.
Venkatakrishnan K, Rader M, Ramanathan RK, Ramalingam S, Chen E, Riordan W, et al. Effect of the CYP3A inhibitor ketoconazole on the pharmacokinetics and pharmacodynamics of bortezomib in patients with advanced solid tumors: a prospective, multicenter, open-label, randomized, two-way crossover drug-drug interaction study. Clin Ther. 2009;31(Pt 2):2444–58.
Sager JE, Yu J, Ragueneau-Majlessi I, Isoherranen N. Physiologically based pharmacokinetic (PBPK) modeling and simulation approaches: a systematic review of published models, applications, and model verification. Drug Metab Dispos. 2015;43(11):1823–37.
Danhof M. Systems pharmacology—towards the modeling of network interactions. Eur J Pharm Sci. 2016;94:4–14.
Kupperman E, Lee EC, Cao Y, Bannerman B, Fitzgerald M, Berger A, et al. Evaluation of the proteasome inhibitor MLN9708 in preclinical models of human cancer. Cancer Res. 2010;70(5):1970–80.
Majetschak M, Sorell LT. Immunological methods to quantify and characterize proteasome complexes: development and application. J Immunol Methods. 2008;334(1–2):91–103.
Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10(7):1093–5.
Cubitt HE, Houston JB, Galetin A. Prediction of human drug clearance by multiple metabolic pathways: integration of hepatic and intestinal microsomal and cytosolic data. Drug Metab Dispos. 2011;39(5):864–73.
Coutant DE, Kulanthaivel P, Turner PK, Bell RL, Baldwin J, Wijayawardana SR, et al. Understanding disease-drug interactions in cancer patients: implications for dosing within the therapeutic window. Clin Pharmacol Ther. 2015;98(1):76–86.
Helsby NA, Lo WY, Sharples K, Riley G, Murray M, Spells K, et al. CYP2C19 pharmacogenetics in advanced cancer: compromised function independent of genotype. Br J Cancer. 2008;99(8):1251–5.
Hosea NA, Collard WT, Cole S, Maurer TS, Fang RX, Jones H, et al. Prediction of human pharmacokinetics from preclinical information: comparative accuracy of quantitative prediction approaches. J Clin Pharmacol. 2009;49(5):513–33.
Bross PF, Kane R, Farrell AT, Abraham S, Benson K, Brower ME, et al. Approval summary for bortezomib for injection in the treatment of multiple myeloma. Clin Cancer Res. 2004;10(12 Pt 1):3954–64.
Zhao P, Ragueneau-Majlessi I, Zhang L, Strong JM, Reynolds KS, Levy RH, et al. Quantitative evaluation of pharmacokinetic inhibition of CYP3A substrates by ketoconazole: a simulation study. J Clin Pharmacol. 2009;49(3):351–9.
An G. Small-molecule compounds exhibiting target-mediated drug disposition (TMDD): a minireview. J Clin Pharmacol. 2017;57(2):137–50.
Levy G. Pharmacologic target-mediated drug disposition. Clin Pharmacol Ther. 1994;56(3):248–52.
Chien JY, Banfield CR, Brazzell RK, Mayer PR, Slattery JT. Saturable tissue binding and imirestat pharmacokinetics in rats. Pharm Res. 1992;9(4):469–73.
Fuchs H, Tillement JP, Urien S, Greischel A, Roth W. Concentration-dependent plasma protein binding of the novel dipeptidyl peptidase 4 inhibitor BI 1356 due to saturable binding to its target in plasma of mice, rats and humans. J Pharm Pharmacol. 2009;61(1):55–62.
An G, Liu W, Katz DA, Marek GJ, Awni W, Dutta S. Population pharmacokinetics of the 11beta-hydroxysteroid dehydrogenase type 1 inhibitor ABT-384 in healthy volunteers following single and multiple dose regimens. Biopharm Drug Dispos. 2014;35(7):417–29.
Khal J, Hine AV, Fearon KC, Dejong CH, Tisdale MJ. Increased expression of proteasome subunits in skeletal muscle of cancer patients with weight loss. Int J Biochem Cell Biol. 2005;37(10):2196–206.
Arlt A, Bauer I, Schafmayer C, Tepel J, Muerkoster SS, Brosch M, et al. Increased proteasome subunit protein expression and proteasome activity in colon cancer relate to an enhanced activation of nuclear factor E2-related factor 2 (Nrf2). Oncogene. 2009;28(45):3983–96.
Jakob C, Egerer K, Liebisch P, Turkmen S, Zavrski I, Kuckelkorn U, et al. Circulating proteasome levels are an independent prognostic factor for survival in multiple myeloma. Blood. 2007;109(5):2100–5.
Funding
This work was funded by Takeda Pharmaceuticals International.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal procedures were approved by the Institutional Animal Care and Use Committee of the contract research organization.
Conflict of Interest
The authors declare that they have no conflicts of interest. All authors were employees of Takeda Pharmaceuticals International Company when the study was performed.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Supplemental Fig. 1
Blood concentration-time profile of bortezomib in monkeys after intravenous injection at a dose of 0.1 mg/kg/dose on Day 1, 4, 8, 11, 22, 25, 29, 32, 43, 46, 50, 53, 64, 67, 71 and 74. Symbols represent the individual observed values (n = 6) and lines indicate the simulated profiles (PNG 7059 kb)
Supplemental Fig. 2
Mean time course of (A) plasma concentration, (B) blood concentration and (C) blood proteasome inhibition of bortezomib after the third dosing in cycle 1 and 2 at a dose of 1.3 mg/m2. Symbols represent the observed values (mean ± S.D., n = 17 for plasma and blood concentration, and n = 15 for blood proteasome inhibition) from study CAN-1001. Dashed lines indicate the simulated profiles using default Bmax value. Solid lines indicate the simulated profiles using 1.4-fold higher Bmax value (PNG 19453 kb)
Rights and permissions
About this article
Cite this article
Iwasaki, S., Zhu, A., Hanley, M. et al. A Translational Physiologically Based Pharmacokinetics/Pharmacodynamics Framework of Target-Mediated Disposition, Target Inhibition and Drug–Drug Interactions of Bortezomib. AAPS J 22, 66 (2020). https://doi.org/10.1208/s12248-020-00448-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-020-00448-x