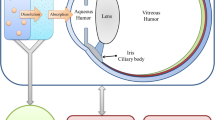
Abstract
FDA’s Orange Book lists 17 currently marketed active pharmaceutical ingredients (API) formulated within ophthalmic suspensions in which a majority has 90% or more of the API undissolved. We used an ocular physiologically based pharmacokinetic (O-PBPK) model to compare a suspension with a solution for ophthalmic products with dexamethasone (Dex) as the model drug. Simulations with a Dex suspension O-PBPK model previously verified in rabbit were used to characterize the consequences of drug clearance mechanism in the precorneal compartment on pharmacokinetic (PK) exposure and to assess the ocular and systemic PK characteristics of ophthalmic suspensions with different strengths or magnitudes of viscosity. O-PBPK-based simulations show that (1) Dex suspension 0.05% has a 2.5- and 5-fold higher AUC in aqueous humor and plasma, respectively, than the Dex saturated solution; (2) strength increase by 5- and 10-fold induces a respective 2.2- and 3.3-fold increase in aqueous humor and 4.4- and 8.6-fold increase in plasma Cmax and AUC; and (3) increasing formulation viscosity (from 1.6 to 75 cP) causes an overall increase in API available for absorption in the cornea resulting in a higher ocular Cmax and AUC with no significant impact on systemic exposure. This research demonstrates that solid particles present in a suspension can not only help to achieve a higher ocular exposure but also unfavorably raise systemic exposure. A model able to correlate formulation changes to both ocular and plasma exposure is a necessary tool to support ocular product development taking into consideration both local efficacy and systemic safety aspects.





Similar content being viewed by others
References
Yellepeddi VK, Palakurthi S. Recent advances in topical ocular drug delivery. J Ocul Pharmacol Ther Off J Assoc Ocul Pharmacol Ther. 2016;32(2):67–82.
Xu X, Al-Ghabeish M, Rahman Z, Krishnaiah YSR, Yerlikaya F, Yang Y, et al. Formulation and process factors influencing product quality and in vitro performance of ophthalmic ointments. Int J Pharm. 2015;493(1–2):412–25.
Patel A, Cholkar K, Agrahari V, Mitra AK. Ocular drug delivery systems: an overview. World J Pharmacol. 2013;2(2):47–64.
US. FDA. Orange Book: approved drug products with therapeutic equivalence evaluations [Internet]. [cited 2018 Dec 15]. Available from: https://www.accessdata.fda.gov/scripts/cder/ob/ Accessed November 13, 2019
Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, et al. PubChem 2019 update: improved access to chemical data. Nucleic Acids Res. 2019 Jan 8;47(D1):D1102–9.
Farkouh A, Frigo P, Czejka M. Systemic side effects of eye drops: a pharmacokinetic perspective. Clin Ophthalmol Auckl NZ. 2016;10:2433–41.
Deng F, Ranta V-P, Kidron H, Urtti A. General pharmacokinetic model for topically administered ocular drug dosage forms. Pharm Res. 2016;33(11):2680–90.
Sieg JW, Robinson JR. Mechanistic studies on transcorneal permeation of pilocarpine. J Pharm Sci. 1976;65(12):1816–22.
Hui HW, Robinson JR. Effect of particle dissolution rate on ocular drug bioavailability. J Pharm Sci. 1986 Mar;75(3):280–7.
Worakul N, Robinson JR. Ocular pharmacokinetics/pharmacodynamics. Eur J Pharm Biopharm. 1997 Jul 1;44(1):71–83.
Le Merdy M, Fan J, Bolger MB, Lukacova V, Spires J, Tsakalozou E, et al. Application of mechanistic ocular absorption modeling and simulation to understand the impact of formulation properties on ophthalmic bioavailability in rabbits: a case study using dexamethasone suspension. AAPS J. 2019 May 20;21(4):65.
US. FDA. MAXIDEX®- dexamethasone ophthalmic suspension Label [Internet]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/013422s045lbl.pdf Accessed November 13, 2019
US. FDA. TOBRADEX® (tobramycin and dexamethasone ophthalmic suspension) Label [Internet]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2003/50592slr032_tobradex_lbl.pdf Accessed November 13, 2019
US. FDA. TOBRADEX® ST (tobramycin / dexamethasone ophthalmic suspension) Label [Internet]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/050818lbl.pdf Accessed November 13, 2019
Lu AT, Frisella ME, Johnson KC. Dissolution modeling: factors affecting the dissolution rates of polydisperse powders. Pharm Res. 1993 Sep;10(9):1308–14.
Patton TF, Robinson JR. Ocular evaluation of polyvinyl alcohol vehicle in rabbits. J Pharm Sci. 1975;64(8):1312–6.
Stephanie C, Wu Y, Darby K, Zheng J, Petrochenko P. Physicochemical characterization of Tobradex and Tobradex ST under physiological conditions. Invest Ophthalmol Vis Sci. 2017 8:4459.
Chockalingam A, Xu L, Stewart S, Le Merdy M, Tsakalozou E, Fan J, et al. Protocol for evaluation of topical ophthalmic drug products in different compartments of fresh eye tissues in a rabbit model. J Pharmacol Toxicol Methods. 2019 1;96:9-14.
Schoenwald RD, Stewart P. Effect of particle size on ophthalmic bioavailability of dexamethasone suspensions in rabbits. J Pharm Sci. 1980 Apr;69(4):391–4.
Sasaki H, Yamamura K, Mukai T, Nishida K, Nakamura J, Nakashima M, et al. Pharmacokinetic prediction of the ocular absorption of an instilled drug with ophthalmic viscous vehicle. Biol Pharm Bull. 2000;23(11):1352–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclaimer
This article reflects the views of the authors and should not be construed to represent the US Food and Drug Administration’s views or policies.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 28 kb)
Rights and permissions
About this article
Cite this article
Le Merdy, M., Tan, ML., Babiskin, A. et al. Physiologically Based Pharmacokinetic Model to Support Ophthalmic Suspension Product Development. AAPS J 22, 26 (2020). https://doi.org/10.1208/s12248-019-0408-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-019-0408-9