Abstract
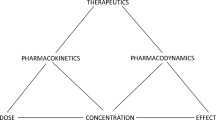
This article provides a dialogue covering an ongoing controversy on the use of clearance versus rate constant approaches for model parameterization when assessing pharmacokinetic (PK) data. It reflects the differences in opinions that can exist among PK experts. Importantly, this discussion extends beyond theoretical arguments to demonstrate how these different approaches impact the analysis and interpretation of data acquired in clinical situations. By not shying away from such dialogues, this article showcases how dissimilarity in well-grounded perspectives can influence how one applies PK and mathematical principles.
Similar content being viewed by others
References
Rowland M, Benet LZ, Graham GG. Clearance concepts in pharmacokinetics. J Pharmacokinet Biopharm. 1973;1(2):123–36.
Mehvar R. Teachers topics. The relationship among pharmacokinetic parameters: effects of altered kinetics on the drug plasma concentration–time profiles. Am J Pharm Educ. 2004;68(2):article 36.
Mehvar R. Interdependency of pharmacokinetic parameters: a chicken-and-egg problem? Not! J Pharm Pharm Sci. 2006;9(1):113–8.
PharmPK Discussion – Clearance vs rate constant (2001). https://www.pharmpk.com/PK01/PK2001055.html. Last visited June 1, 2019.
PharmPK Discussion – Clearance and elimination (2006a). https://www.pharmpk.com/PK06/PK2006120.html. Last visited June 1, 2019.
PharmPK Discussion – What is Clearance? (2006b). https://www.pharmpk.com/PK06/PK2006599.html. Last visited June 1, 2019.
PharmPK Discussion – A question of clearance (2009). https://www.pharmpk.com/PK09/PK2009003.html. Last visited June 1, 2019.
PharmPK Discussion – A question of clearance (2010a). https://www.pharmpk.com/PK10/PK2010002.html. Last visited June 1, 2019.
PharmPK Discussion – Volume of Distribution and Clearance (2010b). https://www.pharmpk.com/PK10/PK2010316.html. Last visited June 1, 2019.
Jelliffe R. Challenges in individualizing drug dosage for intensive care unit patients: is augmented renal clearance what we really want to know? Some suggested management approaches and clinical software tools. Clin Pharmacokinet. 2016;55:897–905. https://doi.org/10.1007/s40262-016-0369-4.
Proost JH. Comment on: “challenges in individualizing drug dosage for intensive care unit patients: is augmented renal clearance what we really want to know? Some suggested management approaches and clinical software tools”. Clin Pharmacokinet. 2017;56:311–2. https://doi.org/10.1007/s40262-016-0480-6.
Jelliffe R and Bayard D. New perspectives in clinical pharmacokinetics-1: the importance of updating the teaching in pharmacokinetics that both clearance and elimination rate constant approaches are mathematically proven equally valid. AAPS J. 2018; 20(2). DOI: https://doi.org/10.1208/s12248-018-0185-x.
Goodwin G, Payne R. Dynamic system identification: experiment design and data analysis. New York: Academic Press; 1977. p. 50. (Theorem 3.4.1)
Jelliffe R, Neely M, editors: “Individualized drug therapy for patients—basic foundations, relevant software, and clinical applications”. Imprint: Academic Press. Release Date: November 15, 2016. Print Book ISBN: 9780128033487.
Jelliffe R, Bayard D, Milman M, Van Guilder M, Schumitzky A. Achieving target goals most precisely using nonparametric compartmental models and “multiple model” design of dosage regimens. Therap Drug Monit. 2000;22:346–53.
Bayard D, Jelliffe R. A Bayesian approach to tracking patients having changing pharmacokinetic parameters. J Pharmacokinet Pharmacodyn. 2004;31:75–107.
Bellman R. Adaptive control processes - a guided tour. Princeton University Press, Princeton, New Jersey; 1961. pp. 255. https://doi.org/10.1002/nav.3800080314.
Neely M, van Guilder M, Yamada W, Schumitzky A, Jelliffe R. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Therap Drug Monit. 2012;34:467–76.
Udy A, Putta M, Shanmugathasana S, Roberts J, Lipman J. Augmented renal clearance in the intensive care unit: an illustrative case series. Int J Antimicrob Agents. 2010;35:606–8.
Mahmoud SH, Shen C. Augmented renal clearance in critical illness: an important consideration in drug dosing. Pharmaceutics. 2017;9(3):36. https://doi.org/10.3390/pharmaceutics9030036.
Haug III MT, Slugg PH. Antibiotic pharmacokinetics. In: Sivak E., Higgins, T., Seiver, A., eds. The High Risk Patient: Management of the Critically Ill. Baltimore: Williams & Wilkin, 1995, pp 1338-64. ISBN 0-683-07785-6
Jelliffe RW, Milman M, Schumitzky A, Bayard D, Van Guilder M. A two-compartment population pharmacokinetic-pharmacodynamic model of digoxin in adults, with implications for dosage. Ther Drug Monit. 2014;36:387–93. https://doi.org/10.1097/FTD.0000000000000023.
Jelliffe R. The role of digitalis pharmacokinetics in converting atrial fibrillation and flutter to regular sinus rhythm. Clin Pharmacokinet. 2014;53:397–407. https://doi.org/10.1007/s40262-014-0141-6.
Bellman R, Astrom XXX. On structural Identifiability. Math Biosci. 1970;7:329–39.
Reuning R, Sams R, Notari. Role of pharmacokinetics in drug dosage adjustment. 1. Pharmacologic effect kinetics and apparent volume of distribution of digoxin. J Clin Pharmacol. 1973;13:127–41.
Jelliffe R. Estimation of creatinine clearance in patients with unstable renal function, without a urine specimen. Am J Nephrol. 2002;22:320–4.
Bustad A, Terziivanov D, Leary R, Port R, Schumitzky A, Jelliffe R. Parametric and nonparametric population methods: their comparative performance in analysing a clinical data set and two Monte Carlo simulation studies. Clin Pharmacokinet. 2006;45:365–83.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This communication reflects the opinion of the author and does not reflect views or policy of the FDA.
Rights and permissions
About this article
Cite this article
Martinez, M.N., Jelliffe, R.W. & Proost, J.H. Expert Discussion of the Role of Rate Constant Versus Clearance Approaches to Define Drug Pharmacokinetics: Theoretical and Clinical Considerations. AAPS J 22, 25 (2020). https://doi.org/10.1208/s12248-019-0407-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-019-0407-x