Abstract
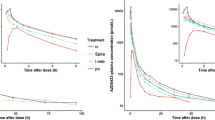
The goal of locally acting inhaled corticosteroids is to achieve distinct pulmonary effects with reduced systemic side effects. The present work using an ex vivo receptor binding model in rats was interested in assessing pulmonary targeting for several commercially available corticosteroids by monitoring receptor occupancies in the lung and systemic organs (liver, kidney, spleen, and brain) after intravenous (IV) injection or intratracheal (IT) instillation of a dry powder administration at a dose of 100 μg/kg. Pulmonary targeting, defined as the difference in cumulative receptor occupancies (AUCE) between the lung and kidney after pulmonary delivery, differed across the investigated corticosteroids (ΔAUCE range, 33 ± 46 to 143 ± 52% *h) with the highest degree found for corticosteroids with high systemic clearance and pronounced lipophilicity (presumably allowing a long pulmonary residence time). Additionally, this study demonstrated differences in the receptor occupancies across systemic organs. Using kidney receptor occupancies as the comparator, liver receptor occupancies were reduced (ΔAUCE range: − 157 ± 43 to 178 ± 42% *h) after IV and IT administration for corticosteroids with high intrinsic clearance, while they were increased for corticosteroid prodrugs due to hepatic activation. Spleen receptor occupancies were increased after IT (ΔAUCE range: 33 ± 35 to 135 ± 28% *h), but not after IV administration. This was especially true for slowly dissolving drugs. Reduced brain uptake was also observed for ciclesonide (CIC) and des-ciclesonide (desCIC), two compounds previously not investigated. In summary, ex vivo receptor binding studies represent a powerful tool to assess the fate of ICSs.





Similar content being viewed by others
Abbreviations
- AMs:
-
alveolar macrophages
- AUCE :
-
accumulative receptor occupancy
- BDP:
-
beclomethasone dipropionate
- BMP:
-
17-beclomethasone monopropionate
- BUD:
-
budesonide
- CIC:
-
ciclesonide
- CL:
-
systemic clearance
- CLint :
-
hepatic intrinsic clearance
- DCs:
-
dendritic cells
- desCIC:
-
des-ciclesonide
- FP:
-
fluticasone propionate
- GR:
-
glucocorticoid receptor
- ICSs:
-
inhaled corticosteroids
- IP:
-
intraperitoneal
- IT:
-
intratracheal
- IV:
-
intravenous
- logP:
-
log octanol-water partition coefficient
- NB:
-
non-specific binding
- PK/PD:
-
pharmacokinetic and pharmacodynamic
- PMSF:
-
phenylmethylsulphonyl fluoride
- P-gp:
-
P-glycoprotein
- RBA:
-
relative binding affinity
- Sol:
-
solubility in water
- TA:
-
triamcinolone acetonide
- TB:
-
total binding
References
Drescher SK, Chen M-J, Bulitta JB, Hochhaus G. Pharmacokinetics and pharmacodynamics of drugs delivered to the lung. In: Hickey AJ, da Rocha SR, editors. Pharmaceutical inhalation aerosol technology. 3rd ed: CRC Press; 2019.
Hochhaus G, Möllmann H, Derendorf H, Gonzalez-Rothi RJ. Pharmacokinetic/pharmacodynamic aspects of aerosol therapy using glucocorticoids as a model. J Clin Pharmacol. 1997;37(10):881–92.
Beato M, Kalimi M, Feigelson P. Correlation between glucocorticoid binding to specific liver cytosol receptors and enzyme induction in vivo. Biochem Biophys Res Commun. 1972;47(6):1464–72.
Hochhaus G, Gonzalez-Rothi RJ, Lukyanov A, Derendorf H, Schreier H, Dalla CT. Assessment of glucocorticoid lung targeting by ex-vivo receptor binding studies in rats. Pharm Res. 1995;12(1):134–7.
Arya V, Issar M, Wang Y, Talton JD, Hochhaus G. Brain permeability of inhaled corticosteroids. J Pharm Pharmacol. 2005;57(9):1159–67.
Mager H, Göller G. Resampling methods in sparse sampling situations in preclinical pharmacokinetic studies. J Pharm Sci. 1998;87(3):372–8.
Bi Y, Deng J, Murry DJ, An G. A whole-body physiologically based pharmacokinetic model of Gefitinib in mice and scale-up to humans. AAPS J. 2016;18(1):228–38.
Choy YB, Prausnitz MR. The rule of five for non-oral routes of drug delivery: ophthalmic, inhalation and transdermal. Pharm Res. 2011;28(5):943–8.
Winkler J, Hochhaus G, Derendorf H. How the lung handles drugs. Proc Am Thorac Soc. 2004;1(4):356–63.
Rohdewald P, Moellmann H, Mueller KM, Hochhaus G. Glucocorticoid receptors in the respiration tract. Bochumer Treff. 1984:223–42.
Stoeck M, Riedel R, Hochhaus G, Häfner D, Masso JM, Schmidt B, et al. In vitro and in vivo anti-inflammatory activity of the new glucocorticoid ciclesonide. J Pharmacol Exp Ther. 2004;309(1):249–58.
Issar M, Sahasranaman S, Buchwald P, Hochhaus G. Differences in the glucocorticoid to progesterone receptor selectivity of inhaled glucocorticoids. Eur Respir J. 2006 Mar;27(3):511–6.
Sakagami M, Kinoshita W, Sakon K, Sato J, Makino Y. Mucoadhesive beclomethasone microspheres for powder inhalation: their pharmacokinetics and pharmacodynamics evaluation. J Control Release Off J Control Release Soc. 2002;80(1–3):207–18.
Boobis AR. Comparative physicochemical and pharmacokinetic profiles of inhaled beclomethasone dipropionate and budesonide. Respir Med. 1998;92(Suppl B):2–6.
van Amerongen IA, De Ronde HAG, Klooster NTM. Physical-chemical characterization of semisolid topical dosage form using a new dissolution system. Int J Pharm. 1992;86(1):9–15.
Feth MP, Volz J, Hess U, Sturm E, Hummel R-P. Physicochemical, crystallographic, thermal, and spectroscopic behavior of crystalline and X-ray amorphous ciclesonide. J Pharm Sci. 2008;97(9):3765–80.
Jain N, Yalkowsky SH. Estimation of the aqueous solubility I: application to organic nonelectrolytes. J Pharm Sci. 2001;90(2):234–52.
Högger P, Rohdewald P. Binding kinetics of fluticasone propionate to the human glucocorticoid receptor. Steroids. 1994;59(10):597–602.
Baumann D, Bachert C, Högger P. Dissolution in nasal fluid, retention and anti-inflammatory activity of fluticasone furoate in human nasal tissue ex vivo. Clin Exp Allergy J Br Soc Allergy Clin Immunol. 2009;39(10):1540–50.
Wang Y. Pharmacokinetic and pharmacodynamic evaluation of beclomethasone dipropionate: University of Florida; 2003.
Talton JD. Pulmonary targeting of inhaled glucocorticoid dry powders: University of Florida; 1999.
Guo Z, Gu Z, Howell SR, Chen K, Rohatagi S, Cai L, et al. Ciclesonide disposition and metabolism: pharmacokinetics, metabolism, and excretion in the mouse, rat, rabbit, and dog. Am J Ther. 2006;13(6):490–501.
Jones RM, Harrison A. A new methodology for predicting human pharmacokinetics for inhaled drugs from oratracheal pharmacokinetic data in rats. Xenobiotica Fate Foreign Compd Biol Syst. 2012;42(1):75–85.
Rojas C, Nagaraja NV, Webb AI, Derendorf H. Microdialysis of triamcinolone acetonide in rat muscle. J Pharm Sci. 2003;92(2):394–7.
Martin LE, Harrison C, Tanner RJ. Metabolism of beclomethasone dipropionate by animals and man. Postgrad Med J. 1975;51(Suppl 4):11–20.
Rohatagi S, Luo Y, Shen L, Guo Z, Schemm C, Huang Y, et al. Protein binding and its potential for eliciting minimal systemic side effects with a novel inhaled corticosteroid, ciclesonide. Am J Ther. 2005;12(3):201–9.
Wu K, Blomgren AL, Ekholm K, Weber B, Edsbaecker S, Hochhaus G. Budesonide and ciclesonide: effect of tissue binding on pulmonary receptor binding. Drug Metab Dispos Biol Fate Chem. 2009;37(7):1421–6.
Taylor S, Harker A. Modification of the ultrafiltration technique to overcome solubility and non-specific binding challenges associated with the measurement of plasma protein binding of corticosteroids. J Pharm Biomed Anal. 2006;41(1):299–303.
Boudinot FD, D’Ambrosio R, Jusko WJ. Receptor-mediated pharmacodynamics of prednisolone in the rat. J Pharmacokinet Biopharm. 1986;14(5):469–93.
Richards ML, Sadée W. In vivo opiate receptor binding of oripavines to mu, delta and kappa sites in rat brain as determined by an ex vivo labeling method. Eur J Pharmacol. 1985;114(3):343–53.
Le S, Gruner JA, Mathiasen JR, Marino MJ, Schaffhauser H́. Correlation between ex vivo receptor occupancy and wakepromoting activity of selective H3 receptor antagonists. J Pharmacol Exp Ther 2008;902–909.
Hochhaus G, Rohdewald P, Möllmann H, Greschuchna D. Identification of glucocorticoid receptors in normal and neoplastic adult human lung. Res Exp Med Z Gesamte Exp Med Einschl Exp Chir. 1983;182(1):71–8.
Ballard PL, Baxter JD, Higgins SJ, Rousseau GG, Tomkins GM. General presence of glucocorticoid receptors in mammalian tissues. Endocrinology. 1974;94(4):998–1002.
Pujols L, Mullol J, Roca-Ferrer J, Torrego A, Xaubet A, Cidlowski JA, et al. Expression of glucocorticoid receptor α- and β-isoforms in human cells and tissues. Am J Physiol-Cell Physiol. 2002;283(4):C1324–31.
Boger E, Ewing P, Eriksson UG, Fihn B-M, Chappell M, Evans N, et al. A novel in vivo receptor occupancy methodology for the glucocorticoid receptor: toward an improved understanding of lung pharmacokinetic/pharmacodynamic relationships. J Pharmacol Exp Ther. 2015;353(2):279–87.
Uller L, Persson CG, Källström L, Erjefält JS. Lung tissue eosinophils may be cleared through luminal entry rather than apoptosis: effects of steroid treatment. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1948–56.
Rohdewald P, Moelhmann H, Hochhaus G. Affinities of glucocorticoids for glucocorticoid receptors in the human lung. Agents Action. 1985;17:290–1.
Hochhaus G. New developments in corticosteroids. Proc Am Thorac Soc. 2004;1(3):269–74.
Derom E, Louis R, Tiesler C, Engelstätter R, Kaufman J-M, Joos GF. Effects of ciclesonide and fluticasone on cortisol secretion in patients with persistent asthma. Eur Respir J. 2009;33(6):1277–86.
Zaidi S, Chen M-J, Lee DT, Neubart E, Ewing P, Miller-Larsson A, et al. Fetal concentrations of budesonide and fluticasone propionate: a study in mice. AAPS J. 2019;21(4):53.
Arredouani MS, Palecanda A, Koziel H, Huang Y-C, Imrich A, Sulahian TH, et al. MARCO is the major binding receptor for unopsonized particles and bacteria on human alveolar macrophages. J Immunol Baltim Md 1950. 2005;175(9):6058–64.
Patel VI, Metcalf JP. Airway macrophage and dendritic cell subsets in the resting human lung. Crit Rev Immunol. 2018;38(4):303–31.
Vogel DYS, Heijnen PDAM, Breur M, de Vries HE, Tool ATJ, Amor S, et al. Macrophages migrate in an activation-dependent manner to chemokines involved in neuroinflammation. J Neuroinflammation. 2014;11:23.
Grayson MH, Ramos MS, Rohlfing MM, Kitchens R, Wang HD, Gould A, et al. Controls for lung dendritic cell maturation and migration during respiratory viral infection. J Immunol. 2007;179(3):1438–48.
Edsbäcker S, Brattsand R. Budesonide fatty-acid esterification: a novel mechanism prolonging binding to airway tissue. Review of available data. Ann Allergy Asthma Immunol Off Publ Am Coll Allergy Asthma Immunol. 2002;88(6):609–16.
Funding
Financial support from 3 M, Takeda (formerly Byk-Gulden), GSK and Astra-Zeneca is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Figure S1:
% Receptor occupied – time profiles for BDP after systemic (IV) and pulmonary (IT) administration. Receptor occupancy in kidney was used as a reference and displayed in each figure. The mean ± SD is given. (GIF 32 kb)
Figure S2:
% Receptor occupied – time profiles for BMP after systemic (IV) and pulmonary (IT) administration. Receptor occupancy in kidney was used as a reference and displayed in each figure. The mean ± SD is given. (GIF 30 kb)
Figure S3:
% Receptor occupied – time profiles for BUD after systemic (IV) and pulmonary (IT) administration. Receptor occupancy in kidney was used as a reference and displayed in each figure. The mean ± SD is given. (GIF 39 kb)
Figure S4:
% Receptor occupied – time profiles for CIC after systemic (IV) and pulmonary (IT) administration. Receptor occupancy in kidney was used as a reference and displayed in each figure. The mean ± SD is given. (GIF 39 kb)
Figure S5:
% Receptor occupied – time profiles for desCIC after systemic (IV) and pulmonary (IT) administration. Receptor occupancy in kidney was used as a reference and displayed in each figure. The mean ± SD is given. (GIF 39 kb)
Figure S6:
% Receptor occupied – time profiles for TA after systemic (IV) and pulmonary (IT) administration. Receptor occupancy in kidney was used as a reference and displayed in each figure. The mean ± SD is given. (GIF 27 kb)
Rights and permissions
About this article
Cite this article
Shao, J., Talton, J., Wang, Y. et al. Quantitative Assessment of Pulmonary Targeting of Inhaled Corticosteroids Using Ex Vivo Receptor Binding Studies. AAPS J 22, 39 (2020). https://doi.org/10.1208/s12248-019-0404-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-019-0404-0