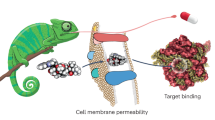
Abstract
The druggability and developability space is rapidly evolving in the post-genomic era. In the past, Lipinski’s rule-of-five (Ro5) emerged and served as a guide for drug-like molecule design for oral delivery in the traditional druggable target space. In contrast, in this new era, a transition is occurring in drug discovery towards novel approaches to bind and modulate challenging biological targets that have led to transformative treatments for patients. Consequently, drugging novel targets using a variety of emerging molecular modalities, namely beyond the Ro5 (bRo5) small molecules (such as protein-protein interaction modulators, protein-targeted chimeras, or PROTACs), peptide/peptidomimetics, and nucleic acid-based modalities, have become a key focus in drug discovery. Herein, the emerging druggability and developability space is discussed side by side to build a general understanding of the potential development challenges of these novel modalities. An overview is provided on the evolving novel targets and molecular modalities, followed by a detailed analysis of the druggability aspects as well as the strategies used to progress drug candidate, and the trending chemistry and formulation strategies used to assess developability.




Similar content being viewed by others
References
Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1(9):727–30.
Abi Hussein H, Geneix C, Petitjean M, Borrel A, Flatters D, Camproux AC. Global vision of druggability issues: applications and perspectives. Drug Discov Today. 2017;22(2):404–15.
Makley LN, Gestwicki JE. Expanding the number of 'druggable' targets: non-enzymes and protein-protein interactions. Chem Biol Drug Des. 2013;81(1):22–32.
Saxena V, Panicucci R, Joshi Y, Garad S. Developability assessment in pharmaceutical industry: an integrated group approach for selecting developable candidates. J Pharm Sci. 2009;98(6):1962–79.
Venkatesh S, Lipper RA. Role of the development scientist in compound lead selection and optimization. J Pharm Sci. 2000;89(2):145–54.
Bak A, Leung D, Barrett SE, Forster S, Minnihan EC, Leithead AW, et al. Physicochemical and formulation developability assessment for therapeutic peptide delivery--a primer. AAPS J. 2015;17(1):144–55.
Mathias NR, Hussain MA. Non-invasive systemic drug delivery: Developability considerations for alternate routes of administration. J Pharm Sci. 2009;99(1):1–20.
Furman JL, Chiu M, Hunter MJ. Early engineering approaches to improve peptide Developability and manufacturability. AAPS J. 2015;17(1):111–20.
Kwong E. Advancing drug discovery: a pharmaceutics perspective. J Pharm Sci. 2015;104(3):865–71.
Ramachander RRN. Molecule and Manufacturability Assessment Leading to Robust Commercial Formulation for Therapeutic Proteins. In: Parag Kolhe MS, Rathore N, editors. Sterile product development AAPS Advances in the Pharmaceutical Sciences Series. 1st ed. New York: Springer-Verlag; 2013. p. 33–45.
Almagro J, Mascioni A. Best practices in assessing the developability of biopharmaceutical candidates. 2015;195–220.
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46(1–3):3–26.
Bayliss MK, Butler J, Feldman PL, Green DV, Leeson PD, Palovich MR, et al. Quality guidelines for oral drug candidates: dose, solubility and lipophilicity. Drug Discov Today. 2016;21(10):1719–27.
Paul SM, Mytelka DS, Dunwiddie CT, Persinger CC, Munos BH, Lindborg SR, et al. How to improve R&D productivity: the pharmaceutical industry's grand challenge. Nat Rev Drug Discov. 2010;9(3):203–14.
Bunnage ME. Getting pharmaceutical R&D back on target. Nat Chem Biol. 2011;7(6):335–9.
Gulcher J, Stefansson K. Population genomics: laying the groundwork for genetic disease modeling and targeting. Clin Chem Lab Med. 1998;36(8):523–7.
Sakharkar MK, Sakharkar KR, Pervaiz S. Druggability of human disease genes. Int J Biochem Cell Biol. 2007;39(6):1156–64.
Lazo JS, Sharlow ER. Drugging Undruggable molecular Cancer targets. Annu Rev Pharmacol Toxicol. 2016;56:23–40.
Leeson PD. Molecular inflation, attrition and the rule of five. Adv Drug Deliv Rev. 2016;101:22–33.
DeGoey DA, Chen HJ, Cox PB, Wendt MD. Beyond the rule of 5: lessons Learned from AbbVie's drugs and compound collection. J Med Chem. 2018;61(7):2636–51.
Shultz MD. Two decades under the influence of the rule of five and the changing properties of approved Oral drugs. J Med Chem. 2019;62(4):1701–14.
Valeur E, Gueret SM, Adihou H, Gopalakrishnan R, Lemurell M, Waldmann H, et al. New modalities for challenging targets in drug discovery. Angew Chem Int Engl. 2017;56(35):10294–323.
Churcher I. Protac-induced protein degradation in drug discovery: breaking the rules or just making new ones? J Med Chem. 2018;61(2):444–52.
Matsson P, Kihlberg J. How big is too big for cell permeability? J Med Chem. 2017;60(5):1662–4.
Matsson P, Doak BC, Over B, Kihlberg J. Cell permeability beyond the rule of 5. Adv Drug Deliv Rev. 2016;101:42–61.
Doak BC, Over B, Giordanetto F, Kihlberg J. Oral druggable space beyond the rule of 5: insights from drugs and clinical candidates. Chem Biol. 2014;21(9):1115–42.
Edmondson SD, Yang B, Fallan C. Proteolysis targeting chimeras (PROTACs) in ‘beyond rule-of-five’ chemical space: recent Progress and future challenges. Bioorg Med Chem Lett. 2019.
Bergström CAS, Porter CJH. Understanding the challenge of beyond-rule-of-5 compounds. Adv Drug Deliv Rev. 2016;101:1–5.
Choo EF, Boggs J, Zhu C, Lubach JW, Catron ND, Jenkins G, et al. The role of lymphatic transport on the systemic bioavailability of the Bcl-2 protein family inhibitors navitoclax (ABT-263) and ABT-199. Drug Metab Dispos. 2014;42(2):207–12.
Neklesa T, Snyder LB, Willard RR, Vitale N, Pizzano J, Gordon DA, et al. ARV-110: An oral androgen receptor PROTAC degrader for prostate cancer. 2019;37(7_suppl):259.
Flanagan J, Qian Y, Gough S, Andreoli M, Bookbinder M, Cadelina G, et al. Abstract P5–04-18: ARV-471, an oral estrogen receptor PROTAC degrader for breast cancer. 2019;79(4 Supplement):P5–04-18-P5–04-18.
Lau JL, Dunn MK. Therapeutic peptides: historical perspectives, current development trends, and future directions. Bioorg Med Chem. 2018;26(10):2700–7.
Henninot A, Collins JC, Nuss JM. The current state of peptide drug discovery: Back to the future? J Med Chem. 2018;61(4):1382–414.
Zorzi A, Deyle K, Heinis C. Cyclic peptide therapeutics: past, present and future. Curr Opin Chem Biol. 2017;38:24–9.
Aguirre TA, Teijeiro-Osorio D, Rosa M, Coulter IS, Alonso MJ, Brayden DJ. Current status of selected oral peptide technologies in advanced preclinical development and in clinical trials. Adv Drug Deliv Rev. 2016;106(Pt B):223–41.
Linzess (linaclotide). Chemistry review. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/202811Orig1s000ChemR.pdf. FDA. 2011.
Tyagi P, Pechenov S, Anand SJ. Oral peptide delivery: translational challenges due to physiological effects. J Control Release. 2018;287:167–76.
Semaglutide Label. FDA;2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/213051s000lbl.pdf. Accessed 27 Sept 2019
Octreotide capsules. Chiasma, Inc.; 2019 [Available from: http://www.chiasmapharma.com/octreotide-capsules. Accessed 10 Sept 2019
Stein CA, Castanotto D. FDA-approved oligonucleotide therapies in 2017. Mol Ther. 2017;25(5):1069–75.
Juliano RL. The delivery of therapeutic oligonucleotides. Nucleic Acids Res. 2016;44(14):6518–48.
Kulkarni JA, Witzigmann D, Chen S, Cullis PR, van der Meel R. Lipid Nanoparticle Technology for Clinical Translation of siRNA Therapeutics. Acc Chem Res. 2019.
Tegsedi (inotersen). Clinical review. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/211172Orig1s000MedR.pdf. Accessed 6 May 2019
Prakash TP, Graham MJ, Yu J, Carty R, Low A, Chappell A, et al. Targeted delivery of antisense oligonucleotides to hepatocytes using triantennary N-acetyl galactosamine improves potency 10-fold in mice. Nucleic Acids Res. 2014;42(13):8796–807.
Maier MA, Jayaraman M, Matsuda S, Liu J, Barros S, Querbes W, et al. Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics. Mol Ther. 2013;21(8):1570–8.
The medicines company presents results from ORION-11, first phase 3 trial of inclisiran, showing durable and potent lowering of LDL-C with twice-yearly dosing. : The Medicines Company. September 2, 2019 [Available from: https://www.themedicinescompany.com/investor/pr/4000045/. Accessed 1 Oct 2019
Arkin MR, Tang Y, Wells JA. Small-molecule inhibitors of protein-protein interactions: progressing toward the reality. Chem Biol. 2014;21(9):1102–14.
Petta I, Lievens S, Libert C, Tavernier J, De Bosscher K. Modulation of protein-protein interactions for the development of novel therapeutics. Mol Ther. 2016;24(4):707–18.
Smith MC, Gestwicki JE. Features of protein-protein interactions that translate into potent inhibitors: topology, surface area and affinity. Expert Rev Mol Med. 2012;14:e16.
Doak BC, Zheng J, Dobritzsch D, Kihlberg J. How beyond rule of 5 drugs and clinical candidates bind to their targets. J Med Chem. 2016;59(6):2312–27.
Kramer SD, Aschmann HE, Hatibovic M, Hermann KF, Neuhaus CS, Brunner C, et al. When barriers ignore the "rule-of-five". Adv Drug Deliv Rev. 2016;101:62–74.
Fischer ES, Park E, Eck MJ, Thoma NH. SPLINTS: small-molecule protein ligand interface stabilizers. Curr Opin Struct Biol. 2016;37:115–22.
Mullard A. Pioneering apoptosis-targeted cancer drug poised for FDA approval. Nat Rev Drug Discov. 2016;15(3):147–9.
Venclexta (venetoclax). Clinical pharmacology and biopharmaceutics review. FDA; 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208573Orig1s000ClinPharmR.pdf. Accessed 6 May 2019
Ottis P, Toure M, Cromm PM, Ko E, Gustafson JL, Crews CM. Assessing different E3 ligases for small molecule induced protein Ubiquitination and degradation. ACS Chem Biol. 2017;12(10):2570–8.
Gu S, Cui D, Chen X, Xiong X, Zhao Y. PROTACs: An Emerging Targeting Technique for Protein Degradation in Drug Discovery. BioEssays. 2018;40(4):e1700247.
Maple HJ, Clayden N, Baron A, Stacey C, Felix R. Developing degraders: principles and perspectives on design and chemical space. MedChemComm. 2019.
Cromm PM, Crews CM. Targeted protein degradation: from chemical biology to drug discovery. Cell Chem Biol. 2017;24(9):1181–90.
Bondeson DP, Mares A, Smith IED, Ko E, Campos S, Miah AH, et al. Catalytic in vivo protein knockdown by small-molecule PROTACs. Nat Chem Biol. 2015;11(8):611–7.
Galdeano C. Drugging the undruggable: targeting challenging E3 ligases for personalized medicine. Future Med Chem. 2017;9(4):347–50.
Neklesa TK, Winkler JD, Crews CM. Targeted protein degradation by PROTACs. Pharmacol Ther. 2017;174:138–44.
Crews CM. Inducing protein degradation as a therapeutic strategy. J Med Chem. 2018;61(2):403–4.
Erak M, Bellmann-Sickert K, Els-Heindl S, Beck-Sickinger AG. Peptide chemistry toolbox – transforming natural peptides into peptide therapeutics. Bioorg Med Chem. 2018;26(10):2759–65.
Pelay-Gimeno M, Glas A, Koch O, Grossmann TN. Structure-based Design of Inhibitors of protein-protein interactions: mimicking peptide binding epitopes. Angew Chem Int Engl. 2015;54(31):8896–927.
Gil-Martin M, Pardo PG, Lopez-Tarruella S, Manso L, Perez-Fidalgo JA, Ademuyiwa FO, et al. Phase I study of the combination of balixafortide (CXCR4 inhibitor) and eribulin in HER2-negative metastatic breast cancer (MBC) patients (pts). 2017;35(15_suppl):2555-.
Lau J, Bloch P, Schäffer L, Pettersson I, Spetzler J, Kofoed J, et al. Discovery of the once-weekly glucagon-like Peptide-1 (GLP-1) analogue Semaglutide. J Med Chem. 2015;58(18):7370–80.
Yu M, Benjamin MM, Srinivasan S, Morin EE, Shishatskaya EI, Schwendeman SP, et al. Battle of GLP-1 delivery technologies. Adv Drug Deliv Rev. 2018;130:113–30.
Zarei-Ghanavati S, Alizadeh R, Deng S. Topical interferon alpha-2b for treatment of noninvasive ocular surface squamous neoplasia with 360° limbal involvement. J Ophthalmic Vis Res. 2014;9(4):423–6.
McCormack PL. Linaclotide: a review of its use in the treatment of irritable bowel syndrome with constipation. Drugs. 2014;74(1):53–60.
Schwochert J, Turner R, Thang M, Berkeley RF, Ponkey AR, Rodriguez KM, et al. Peptide to Peptoid substitutions increase cell permeability in cyclic Hexapeptides. Org Lett. 2015;17(12):2928–31.
Whitty A, Zhong M, Viarengo L, Beglov D, Hall DR, Vajda S. Quantifying the chameleonic properties of macrocycles and other high-molecular-weight drugs. Drug Discov Today. 2016;21(5):712–7.
Over B, Matsson P, Tyrchan C, Artursson P, Doak BC, Foley MA, et al. Structural and conformational determinants of macrocycle cell permeability. Nat Chem Biol. 2016;12(12):1065–74.
Rezai T, Bock JE, Zhou MV, Kalyanaraman C, Lokey RS, Jacobson MP. Conformational flexibility, internal hydrogen bonding, and passive membrane permeability: successful in silico prediction of the relative permeabilities of cyclic peptides. J Am Chem Soc. 2006;128(43):14073–80.
Knudsen LB, Lau J. The Discovery and Development of Liraglutide and Semaglutide. 2019;10(155).
Buckley ST, Bækdal TA, Vegge A, Maarbjerg SJ, Pyke C, Ahnfelt-Rønne J, et al. Transcellular stomach absorption of a derivatized glucagon-like peptide-1 receptor agonist. 2018;10(467):eaar7047.
Tuvia S, Pelled D, Marom K, Salama P, Levin-Arama M, Karmeli I, et al. A novel suspension formulation enhances intestinal absorption of macromolecules via transient and reversible transport mechanisms. Pharm Res. 2014;31(8):2010–21.
Mullard A. FDA approves landmark RNAi drug. Nat Rev Drug Discov. 2018;17:613.
Khorkova O, Hsiao J, Wahlestedt C. Basic biology and therapeutic implications of lncRNA. Adv Drug Deliv Rev. 2015;87:15–24.
Juliano RL, Ming X, Carver K, Laing B. Cellular uptake and intracellular trafficking of oligonucleotides: implications for oligonucleotide pharmacology. Nucleic Acid Ther. 2014;24(2):101–13.
Abe K, Fujiyoshi Y. Cryo-electron microscopy for structure analyses of membrane proteins in the lipid bilayer. Curr Opin Struct Biol. 2016;39:71–8.
Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902.
Eckstein F. Phosphorothioates, essential components of therapeutic oligonucleotides. Nucleic Acid Ther. 2014;24(6):374–87.
Agrawal S, Goodchild J, Civeira MP, Thornton AH, Sarin PS, Zamecnik PC. Oligodeoxynucleoside phosphoramidates and phosphorothioates as inhibitors of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1988;85(19):7079–83.
Dirin M, Winkler J. Influence of diverse chemical modifications on the ADME characteristics and toxicology of antisense oligonucleotides. Expert Opin Biol Ther. 2013;13(6):875–88.
Stanton MG, Colletti SL. Medicinal chemistry of siRNA delivery. J Med Chem. 2010;53(22):7887–901.
Kenski DM, Cooper AJ, Li JJ, Willingham AT, Haringsma HJ, Young TA, et al. Analysis of acyclic nucleoside modifications in siRNAs finds sensitivity at position 1 that is restored by 5′-terminal phosphorylation both in vitro and in vivo. Nucleic Acids Res. 2010;38(2):660–71.
Parmar R, Willoughby JL, Liu J, Foster DJ, Brigham B, Theile CS, et al. 5′-(E)-Vinylphosphonate: a stable phosphate mimic can improve the RNAi activity of siRNA-GalNAc conjugates. Chembiochem. 2016;17(11):985–9.
Lam JK, Chow MY, Zhang Y, Leung SW. siRNA Versus miRNA as Therapeutics for Gene Silencing. Mol Ther Nucleic Acids. 2015;4:e252.
Stephenson ML, Zamecnik PC. Inhibition of Rous sarcoma viral RNA translation by a specific oligodeoxyribonucleotide. Proc Natl Acad Sci U S A. 1978;75(1):285–8.
Weidner DA, Valdez BC, Henning D, Greenberg S, Busch H. Phosphorothioate oligonucleotides bind in a non sequence-specific manner to the nucleolar protein C23/nucleolin. FEBS Lett. 1995;366(2–3):146–50.
Aartsma-Rus A. New momentum for the field of oligonucleotide therapeutics. Mol Ther. 2016;24(2):193–4.
Verma A. Recent advances in antisense oligonucleotide therapy in genetic neuromuscular diseases. Ann Indian Acad Neurol. 2018;21(1):3–8.
Shen X, Corey DR. Chemistry, mechanism and clinical status of antisense oligonucleotides and duplex RNAs. Nucleic Acids Res. 2018;46(4):1584–600.
Yu RZ, Graham MJ, Post N, Riney S, Zanardi T, Hall S, et al. Disposition and pharmacology of a GalNAc3-conjugated ASO targeting human lipoprotein (a) in mice. Mol Ther Nucleic Acids. 2016;5:e317.
Willoughby JLS, Chan A, Sehgal A, Butler JS, Nair JK, Racie T, et al. Evaluation of GalNAc-siRNA conjugate activity in pre-clinical animal models with reduced Asialoglycoprotein receptor expression. Mol Ther. 2018;26(1):105–14.
Scharner J, Qi S, Rigo F, Bennett CF, Krainer AR. Delivery of GalNAc-conjugated splice-switching ASOs to non-hepatic cells through ectopic expression of Asialoglycoprotein receptor. Mol Ther Nucleic Acids. 2019;16:313–25.
Egli M, Manoharan M. Re-engineering RNA molecules into therapeutic agents. Acc Chem Res. 2019;52(4):1036–47.
Khvorova A, Watts JK. The chemical evolution of oligonucleotide therapies of clinical utility. Nat Biotechnol. 2017;35(3):238–48.
Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391(6669):806–11.
Zimmermann TS, Lee AC, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, et al. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441(7089):111–4.
Alnylam Pharmaceuticals I. Methods of treating transthyretin (TTR) mediated amyloidosis. US10060921B2. 2018.
Sabnis S, Kumarasinghe ES, Salerno T, Mihai C, Ketova T, Senn JJ, et al. A novel amino lipid series for mRNA delivery: improved Endosomal escape and sustained pharmacology and safety in non-human primates. Mol Ther. 2018;26(6):1509–19.
Givosiran Label. FDA; 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/0212194s000lbl.pdf. Accessed 22 Nov 2019
Gan LM, Lagerstrom-Fermer M, Carlsson LG, Arfvidsson C, Egnell AC, Rudvik A, et al. Intradermal delivery of modified mRNA encoding VEGF-A in patients with type 2 diabetes. Nat Commun. 2019;10(1):871.
Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol. 2018;16(1):71.
Landis MS, Bhattachar S, Yazdanian M, Morrison J. Commentary: why pharmaceutical scientists in early drug discovery are critical for influencing the design and selection of optimal drug candidates. AAPS PharmSciTech. 2018;19(1):1–10.
Bhattachar SN, Bender DM, Sweetana SA, Wesley JA. Discovery formulations: approaches and practices in early preclinical development. In: Templeton AC, Byrn SR, Haskell RJ, Prisinzano TE, editors. Discovering and developing molecules with optimal drug-like properties. New York: Springer New York; 2015. p. 49–94.
Yang NJ, Hinner MJ. Getting across the cell membrane: an overview for small molecules, peptides, and proteins. Methods Mol Biol. 2015;1266:29–53.
Transforming the market. Rani Therapeutics; 2019. Available from: https://www.ranitherapeutics.com/. Accessed 1 Oct 2019
Abramson A, Caffarel-Salvador E, Khang M, Dellal D, Silverstein D, Gao Y, et al. An ingestible self-orienting system for oral delivery of macromolecules. Science. 2019;363(6427):611–5.
Acknowledgments
The authors thank Carolyn A. Heusser at Novartis Institutes for BioMedical Research for carefully reviewing the manuscripts and providing helpful comments for revision. The authors also thank Sara Danforth at Novartis Institutes for BioMedical Research for her key assistance on creating the figures.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 2244 kb)
Rights and permissions
About this article
Cite this article
Yang, W., Gadgil, P., Krishnamurthy, V.R. et al. The Evolving Druggability and Developability Space: Chemically Modified New Modalities and Emerging Small Molecules. AAPS J 22, 21 (2020). https://doi.org/10.1208/s12248-019-0402-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-019-0402-2