Abstract
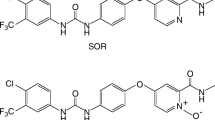
The multikinase inhibitor sorafenib (SOR) is used to treat patients with hepatocellular and renal carcinomas. SOR undergoes CYP-mediated biotransformation to a pharmacologically active N-oxide metabolite (SNO) that has been shown to accumulate to varying extents in individuals. Kinase inhibitors like SOR are frequently coadministered with a range of other drugs to improve the efficacy of anticancer drug therapy and to treat comorbidities. Recent evidence has suggested that SNO is more effective than SOR as an inhibitor of CYP3A4-mediated midazolam 1′-hydroxylation. CYP2D6 is also reportedly inhibited by SOR. The present study assessed the possibility that SNO might contribute to CYP2D6 inhibition. The inhibition kinetics of CYP2D6-mediated dextromethorphan O-demethylation were analyzed in human hepatic microsomes, with SNO found to be ~ 19-fold more active than SOR (Kis 1.8 ± 0.3 μM and 34 ± 11 μM, respectively). Molecular docking studies of SOR and SNO were undertaken using multiple crystal structures of CYP2D6. Both molecules mediated interactions with key amino acid residues in putative substrate recognition sites of CYP2D6. However, a larger number of H-bonding interactions was noted between the N-oxide moiety of SNO and active site residues that account for its greater inhibition potency. These findings suggest that SNO has the potential to contribute to pharmacokinetic interactions involving SOR, perhaps in those individuals in whom SNO accumulates.





Similar content being viewed by others
Abbreviations
- AUC:
-
area under the serum concentration versus time curve
- C max :
-
maximal serum concentration
- CYP:
-
cytochrome P450
- DDI:
-
drug-drug interaction
- SOR:
-
sorafenib
- SNO:
-
sorafenib N-oxide
- SRS:
-
substrate recognition site
References
Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. New Engl J Med. 2007;356:125–34. https://doi.org/10.1056/NEJMoa060655.
Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–8. https://doi.org/10.1158/0008-5472.CAN-06-1377.
Awada A, Hendlisz A, Christensen O, Lathia CD, Bartholomeus S, Lebrun F, et al. Phase I trial to investigate the safety, pharmacokinetics and efficacy of sorafenib combined with docetaxel in patients with advanced refractory solid tumours. Eur J Cancer. 2012;48:465–74. https://doi.org/10.1016/j.ejca.2011.12.026.
Lathia C, Lettieri J, Cihon F, Gallentine M, Radtke M, Sundaresan P. Lack of effect of ketoconazole-mediated CYP3A inhibition on sorafenib clinical pharmacokinetics. Cancer Chemother Pharmacol. 2006;57:685–92. https://doi.org/10.1007/s00280-005-0068-6.
Ghassabian S, Rawling T, Zhou F, Doddareddy MR, Tattam BN, Hibbs DE, et al. Role of human CYP3A4 in the biotransformation of sorafenib to its major oxidized metabolites. Biochem Pharmacol. 2012;84:215–23. https://doi.org/10.1016/j.bcp.2012.04.001.
Strumberg D, Clark JW, Awada A, Moore MJ, Richly H, Hendlisz A, et al. Safety, pharmacokinetics, and preliminary antitumor activity of sorafenib: a review of four phase I trials in patients with advanced refractory solid tumors. Oncologist. 2007;12:426–37. https://doi.org/10.1634/theoncologist.12-4-426.
Minami H, Kawada K, Ebi H, Kitagawa K, Kim YI, Araki K, et al. Phase I and pharmacokinetic study of sorafenib, an oral multikinase inhibitor, in Japanese patients with advanced refractory solid tumors. Cancer Sci. 2008;99:1492–8. https://doi.org/10.1111/j.1349-7006.2008.00837.x.
Inaba H, Rubnitz JE, Coustan-Smith E, Li L, Furmanski BD, Mascara GP, et al. Phase I pharmacokinetic and pharmacodynamic study of the multikinase inhibitor sorafenib in combination with clofarabine and cytarabine in pediatric relapsed/refractory leukemia. J Clin Oncol. 2011;29:3293–300. https://doi.org/10.1200/JCO.2011.34.7427.
Ferrario C, Strepponi I, Esfahani K, Charamis H, Langleben A, Scarpi E, et al. Phase I/II trial of sorafenib in combination with vinorelbine as first-line chemotherapy for metastatic breast cancer. PLoS One. 2016;11:e0167906. https://doi.org/10.1371/journal.pone.0167906.
Ghassabian S, Gillani TB, Rawling T, Crettol S, Nair PC, Murray M. Sorafenib N-oxide is an inhibitor of human hepatic CYP3A4. AAPS J. 2019;21:15. https://doi.org/10.1208/s12248-018-0262-1.
Gillani T, Rawling T, Murray M. Cytochrome P450-mediated biotransformation of sorafenib and its N-oxide metabolite: implications for cell viability and human toxicity. Chem Res Toxicol. 2015;28:92–102. https://doi.org/10.1021/tx500373g.
Murray M, Wilkinson CF, Marcus C, Dube CE. Structure-activity relationships in the interactions of alkoxymethylenedioxybenzene derivatives with rat hepatic microsomal mixed-function oxidases in vivo. Mol Pharmacol. 1983;24:129–36.
Cantrill E, Murray M, Mehta I, Farrell GC. Down-regulation of the male-specific steroid 16α-hydroxylase, cytochrome P-450UT-A, in male rats with portal bypass: relevance to estradiol accumulation and impaired drug metabolism in hepatic cirrhosis. J Clin Invest. 1989;83:1211–6. https://doi.org/10.1172/JCI114003.
Ghassabian S, Chetty M, Tattam BN, Glen J, Rahme J, Stankovic Z, et al. A high-throughput assay using liquid chromatography/tandem mass spectrometry for simultaneous in vivo phenotyping of five major cytochrome P450 enzymes in patients. Ther Drug Monit. 2009;31:239–46. https://doi.org/10.1097/FTD.0b013e318197e1bf.
Murray M. Metabolite intermediate complexation of microsomal cytochrome P450 2C11 in male rat liver by nortriptyline. Mol Pharmacol. 1992;42:931–8.
Johnston RA, Rawling T, Chan T, Zhou F, Murray M. Selective inhibition of human solute carrier transporters by multikinase inhibitors. Drug Metab Dispos. 2014;42:1851–7. https://doi.org/10.1124/dmd.114.059097.
von Moltke LL, Greenblatt DJ, Cotreau-Bibbo MM, Duan SX, Harmatz JS, Shader RI. Inhibition of desipramine hydroxylation in vitro by serotonin-reuptake-inhibitor antidepressants, and by quinidine and ketoconazole: a model system to predict drug interactions in vivo. J Pharmacol Exp Ther. 1994;268:1278–83.
Fiser A, Sali A. ModLoop: automated modeling of loops in protein structures. Bioinformatics. 2003;19:2500–1. https://doi.org/10.1093/bioinformatics/btg362.
Gasteiger J, Marsili M. Iterative partial equalization of orbital electronegativity—a rapid access to atomic charges. Tetrahedron. 1980;36:3219–28. https://doi.org/10.1016/0040-4020(80)80168-2.
Powell MJD. Restart procedures for the conjugate gradient method. Math Program. 1977;12:241–54. https://doi.org/10.1007/BF01593790.
Clark M, Cramer RD III, Van Opdenbosch N. Validation of the general purpose tripos 5.2 force field. J Comput Chem. 1989;10:982–1012. https://doi.org/10.1002/jcc.540100804.
Jain AN. Surflex: fully automatic flexible molecular docking using a molecular similarity-based search engine. J Med Chem. 2003;46:499–511. https://doi.org/10.1021/jm020406h.
Nair PC, McKinnon RA, Miners JO. Computational prediction of the site(s) of metabolism and binding modes of protein kinase inhibitors metabolized by CYP3A4. Drug Metab Dispos. 2019;47:616–31. https://doi.org/10.1124/dmd.118.085167.
Pham TA, Jain AN. Parameter estimation for scoring protein-ligand interactions using negative training data. J Med Chem. 2006;49:5856–68. https://doi.org/10.1021/jm050040j.
Dayer P, Leemann T, Striberni R. Dextromethorphan O-demethylation in liver microsomes as a prototype reaction to monitor cytochrome P-450 db1 activity. Clin Pharmacol Ther. 1989;45:34–40. https://doi.org/10.1038/clpt.1989.6.
Marcus CB, Murray M, Wilkinson CF. Spectral and inhibitory interactions of methylenedioxyphenyl and related compounds with purified isozymes of cytochrome P-450. Xenobiotica. 1985;15:351–62. https://doi.org/10.3109/00498258509045370.
Butler AM, Murray M. Inhibition and inactivation of constitutive cytochromes P450 in rat liver by parathion. Mol Pharmacol. 1993;43:902–8.
Ortiz de Montellano PR, Correia MA. Suicidal destruction of cytochrome P-450 during oxidative drug metabolism. Annu Rev Pharmacol Toxicol. 1983;23:481–503. https://doi.org/10.1146/annurev.pa.23.040183.002405.
He K, Iyer KR, Hayes RN, Sinz MW, Woolf T, Hollenberg PF. Inactivation of cytochrome P450 3A4 by bergamottin, a component of grapefruit juice. Chem Res Toxicol. 1998;11:252–9. https://doi.org/10.1021/tx970192k.
Stupans I, Murray M, Kirlich A, Tuck K, Hayball P. Inactivation of cytochrome P450 by the food-derived complex phenol oleuropein. Food Chem Toxicol. 2001;39:1119–24. https://doi.org/10.1016/S0278-6915(01)00060-6.
Murray M. Complexation of cytochrome P-450 isoenzymes in hepatic microsomes from SKF 525-A-induced rats. Arch Biochem Biophys. 1988;262:381–8. https://doi.org/10.1016/0003-9861(88)90202-0.
Murray M, Field SL. Inhibition and metabolite complexation of rat hepatic microsomal cytochrome P450 by tricyclic antidepressants. Biochem Pharmacol. 1992;43:2065–71. https://doi.org/10.1016/0006-2952(92)90163-D.
Murray M. In vitro effects of quinoline derivatives on cytochrome P-450 and aminopyrine N-demethylase activity in rat hepatic microsomes. Biochem Pharmacol. 1984;33:3277–81. https://doi.org/10.1016/0006-2952(84)90090-X.
Nemeroff CB, DeVane CL, Pollock BG. Newer antidepressants and the cytochrome P450 system. Am J Psychiatr. 1996;153:311–20. https://doi.org/10.1176/ajp.153.3.311.
Ohyama K, Nakajima M, Suzuki M, Shimada N, Yamazaki H, Yokoi T. Inhibitory effects of amiodarone and its N-deethylated metabolite on human cytochrome P450 activities: prediction of in vivo drug interactions. Br J Clin Pharmacol. 2000;49:244–53. https://doi.org/10.1046/j.1365-2125.2000.00134.x.
Giri P, Naidu S, Patel N, Patel H, Srinivas NR. Evaluation of in vitro cytochrome P450 inhibition and in vitro fate of structurally diverse N-oxide metabolites: case studies with clozapine, levofloxacin, roflumilast, voriconazole and zopiclone. Eur J Drug Metab Pharmacokinet. 2017;42:677–88. https://doi.org/10.1007/s13318-016-0385-7.
Rodrigues AD. Measurement of human liver microsomal cytochrome P450 2D6 activity using [O-methyl-14C] dextromethorphan as substrate. Methods Enzymol. 1996;272:186–95. https://doi.org/10.1016/s0076-6879(96)72023-2.
Fowler S, Zhang H. In vitro evaluation of reversible and irreversible cytochrome P450 inhibition: current status on methodologies and their utility for predicting drug-drug interactions. AAPS J. 2008;10:410–23. https://doi.org/10.1208/s12248-008-9042-7.
Gotoh O. Substrate recognition sites in cytochrome P450 family 2 (CYP2) proteins inferred from comparative analyses of amino acid and coding nucleotide sequences. J Biol Chem. 1992;267:83–90.
Bapiro TE, Hasler JA, Ridderström M, Masimirembwa CM. The molecular and enzyme kinetic basis for the diminished activity of the cytochrome P450 2D6.17 (CYP2D6.17) variant. Potential implications for CYP2D6 phenotyping studies and the clinical use of CYP2D6 substrate drugs in some African populations. Biochem Pharmacol. 2002;64:1387–98. https://doi.org/10.1016/s0006-2952(02)01351-5.
Venhorst J, ter Laak AM, Commandeur JN, Funae Y, Hiroi T, Vermeulen NP. Homology modeling of rat and human cytochrome P450 2D (CYP2D) isoforms and computational rationalization of experimental ligand-binding specificities. J Med Chem. 2003;46:74–86. https://doi.org/10.1021/jm0209578.
Rowland P, Blaney FE, Smyth MG, Jones JJ, Leydon VR, Oxbrow AK, et al. Crystal structure of human cytochrome P450 2D6. J Biol Chem. 2006;281:7614–22. https://doi.org/10.1074/jbc.M511232200.
Maréchal JD, Kemp CA, Roberts GC, Paine MJ, Wolf CR, Sutcliffe MJ. Insights into drug metabolism by cytochromes P450 from modelling studies of CYP2D6-drug interactions. Br J Pharmacol. 2008;153(Suppl 1):S82–9. https://doi.org/10.1038/sj.bjp.0707570.
Ferrara P, Gohlke H, Price DJ, Klebe G, Brooks CL 3rd. Assessing scoring functions for protein-ligand interactions. J Med Chem. 2004;47:3032–47. https://doi.org/10.1021/jm030489h.
Clive S, Woo MM, Nydam T, Kelly L, Squier M, Kagan M. Characterizing the disposition, metabolism, and excretion of an orally active pan-deacetylase inhibitor, panobinostat, via trace radiolabeled 14C material in advanced cancer patients. Cancer Chemother Pharmacol. 2012;70:513–22. https://doi.org/10.1007/s00280-012-1940-9.
Knieling F, Waldner MJ, Goertz RS, Strobel D. Quantification of dynamic contrast-enhanced ultrasound in HCC: prediction of response to a new combination therapy of sorafenib and panobinostat in advanced hepatocellular carcinoma. BMJ Case Rep. 2012;2012:bcr2012007576. https://doi.org/10.1136/bcr-2012-007576.
Gahr S, Wissniowski T, Zopf S, Strobel D, Pustowka A, Ocker M. Combination of the deacetylase inhibitor panobinostat and the multi-kinase inhibitor sorafenib for the treatment of metastatic hepatocellular carcinoma - review of the underlying molecular mechanisms and first case report. J Cancer. 2012;3:158–65. https://doi.org/10.7150/jca.4211.
Tsilimigras DI, Ntanasis-Stathopoulos I, Moris D, Spartalis E, Pawlik TM. Histone deacetylase inhibitors in hepatocellular carcinoma: a therapeutic perspective. Surg Oncol. 2018;27:611–8. https://doi.org/10.1016/j.suronc.2018.07.015.
Uttamsingh V, Lu C, Miwa G, Gan LS. Relative contributions of the five major human cytochromes P450, 1A2, 2C9, 2C19, 2D6, and 3A4, to the hepatic metabolism of the proteasome inhibitor bortezomib. Drug Metab Dispos. 2005;33:1723–8. https://doi.org/10.1124/dmd.105.005710.
Massarweh S, Moss J, Wang C, Romond E, Slone S, Weiss H, et al. Impact of adding the multikinase inhibitor sorafenib to endocrine therapy in metastatic estrogen receptor-positive breast cancer. Future Oncol. 2014;10:2435–48. https://doi.org/10.2217/fon.14.99.
Miller AA, Murry DJ, Owzar K, Hollis DR, Kennedy EB, Abou-Alfa G, et al. Phase I and pharmacokinetic study of sorafenib in patients with hepatic or renal dysfunction: CALGB 60301. J Clin Oncol. 2009;27:1800–5. https://doi.org/10.1200/JCO.2008.20.0931.
Flaherty KT, Lathia C, Frye RF, Schuchter L, Redlinger M, Rosen M, et al. Interaction of sorafenib and cytochrome P450 isoenzymes in patients with advanced melanoma: a phase I/II pharmacokinetic interaction study. Cancer Chemother Pharmacol. 2011;68:1111–8. https://doi.org/10.1007/s00280-011-1585-0.
Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013;138:103–41. https://doi.org/10.1016/j.pharmthera.2012.12.007.
Murray M, Gillani TB, Ghassabian S, Edwards RJ, Rawling T. Differential effects of hepatic cirrhosis on the intrinsic clearances of sorafenib and imatinib by CYPs in human liver. Eur J Pharm Sci. 2018;114:55–63. https://doi.org/10.1016/j.ejps.2017.12.003.
Nair PC, McKinnon RA, Miners JO. Cytochrome P450 structure-function: insights from molecular dynamics simulations. Drug Metab Rev. 2016;48:434–52. https://doi.org/10.1080/03602532.2016.1178771.
Acknowledgments
Technical contributions in aspects of the study from Dr. S. Cui and Ms. K Bourget are also acknowledged. The supply of the human liver samples used in this study by Dr. J George is also gratefully acknowledged.
Funding
This study is financially supported by the Cancer Council NSW (grants RG09-14 and IG11-33). PCN is supported by the Flinders Centre for Innovation in Cancer (FCIC) and Flinders Medical Centre (FMC) Foundation through an Early Career Research Grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Murray, M., Gillani, T.B., Rawling, T. et al. Inhibition of Hepatic CYP2D6 by the Active N-Oxide Metabolite of Sorafenib. AAPS J 21, 107 (2019). https://doi.org/10.1208/s12248-019-0374-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-019-0374-2