Abstract
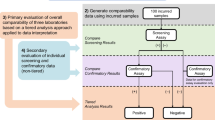
Anti-drug antibody (ADA) assay selectivity is evaluated during assay validation to assess the potential for individual matrices to interfere with detection of ADA. While current EMA and FDA guideline documents suggest comparative analysis with and without matrix, they do not provide specific recommendations on the acceptance criteria such as an acceptable percent positive control (PC) recovery range or positive rate. Industry has adopted an approach where recovery of PC spiked sample is expected to fall within ± 20% (80 to 120%) vs. that for the PC material spiked in negative control matrix or assay buffer. Here, it is proposed that ADA assay selectivity evaluated using a qualitative assessment of PC recovery vs. a PK-like quantitative method may be more appropriate. The PC recovery test should focus on the reliability of the method to detect the low PC level in individual samples and avoid false-negative ADA reporting. Therefore, it is proposed that assessment of high PC level as well as the assessment of quantitative percent recovery (within ± 20%) should not be included in the test. The recovery test may be viewed as acceptable should a pre-selected number of individual samples (for example at least 8 or 9 out of 10) prepared at the low PC concentration of the assay score as ADA positive.



Similar content being viewed by others
References
Committee for Medicinal Products for Human Use C. Guideline on immunogenicity assessment of therapeutic proteins 2017; Available from: https://www.ema.europa.eu/documents/scientific-guideline/guideline-immunogenicity-assessment-therapeutic-proteins-revision-1_en.pdf. Accessed 05 June 2019.
FDA. Immunogenicity testing of therapeutic protein products —developing and validating assays for anti-drug antibody detection. Guidance for industry. U.S. Department of Health and Human Services Food and Drug Administration. Center for Drug Evaluation and Research (CDER). Center for Biologics Evaluation and Research (CBER); 2019 [cited 2019]; January 2019:[Available from: https://www.fda.gov/ucm/groups/fdagov-public/@fdagov-drugs-gen/documents/document/ucm629728.pdf.
Shankar G, Devanarayan V, Amaravadi L, Barrett YC, Bowsher R, Finco-Kent D, et al. Recommendations for the validation of immunoassays used for detection of host antibodies against biotechnology products. J Pharm Biomed Anal. 2008;48(5):1267–81. https://doi.org/10.1016/j.jpba.2008.09.020.
Gorovits B, McNally J, Fiorotti C, Leung S. Protein-based matrix interferences in ligand-binding assays. Bioanalysis. 2014;6(8):1131–40. https://doi.org/10.4155/bio.14.56.
Manning MS, Kroenke MA, Lee SA, Harrison SE, Hoofring SA, Mytych DT, et al. Assay signal as an alternative to titer for assessment of magnitude of an antidrug antibody response. Bioanalysis. 2017;9(23):1849–58. https://doi.org/10.4155/bio-2017-0185.
Liang M, Klakamp SL, Funelas C, Lu H, Lam B, Herl C, et al. Detection of high- and low-affinity antibodies against a human monoclonal antibody using various technology platforms. Assay and drug development technologies. 2007;5(5):655–62. https://doi.org/10.1089/adt.2007.089.
Findlay JW, Smith WC, Lee JW, Nordblom GD, Das I, DeSilva BS, et al. Validation of immunoassays for bioanalysis: a pharmaceutical industry perspective. J Pharm Biomed Anal. 2000;21(6):1249–73.
Bourdage JS, Cook CA, Farrington DL, Chain JS, Konrad RJ. An affinity capture elution (ACE) assay for detection of anti-drug antibody to monoclonal antibody therapeutics in the presence of high levels of drug. J Immunol Methods. 2007;327(1–2):10–7.
Mikulskis A, Yeung D, Subramanyam M, Amaravadi L. Solution ELISA as a platform of choice for development of robust, drug tolerant immunogenicity assays in support of drug development. J Immunol Methods. 2011;365(1–2):38–49. https://doi.org/10.1016/j.jim.2010.11.011.
Stubenrauch K, Wessels U, Essig U, Vogel R, Schleypen J. Evaluation of a generic immunoassay with drug tolerance to detect immune complexes in serum samples from cynomolgus monkeys after administration of human antibodies. J Pharm Biomed Anal. 2010;52(2):249–54.
Xue L, Clements-Egan A, Amaravadi L, Birchler M, Gorovits B, Liang M, et al. Recommendations for the assessment and management of pre-existing drug-reactive antibodies during biotherapeutic development. AAPS J. 2017;19(6):1576–86. https://doi.org/10.1208/s12248-017-0153-x.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gorovits, B., Roldan, M.A., Baltrukonis, D. et al. Anti-drug Antibody Assay Validation: Improved Reporting of the Assay Selectivity via Simpler Positive Control Recovery Data Analysis. AAPS J 21, 76 (2019). https://doi.org/10.1208/s12248-019-0347-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-019-0347-5