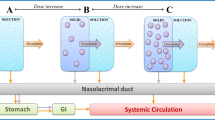
Abstract
Developing mathematical models to predict changes in ocular bioavailability and pharmacokinetics due to differences in the physicochemical properties of complex topical ophthalmic suspension formulations is important in drug product development and regulatory assessment. Herein, we used published FDA clinical pharmacology review data, in-house, and literature rabbit pharmacokinetic data generated for dexamethasone ophthalmic suspensions to demonstrate how the mechanistic Ocular Compartmental Absorption and Transit model by GastroPlus™ can be used to characterize ocular drug pharmacokinetic performance in rabbits for suspension formulations. This model was used to describe the dose-dependent (0.01 to 0.1%) non-linear pharmacokinetic in ocular tissues and characterize the impact of viscosity (1.67 to 72.9 cP) and particle size (5.5 to 22 μm) on in vivo ocular drug absorption and disposition. Parameter sensitivity analysis (hypothetical suspension particle size: 1 to 10 μm, viscosity: 1 to 100 cP) demonstrated that the interplay between formulation properties and physiological clearance through drainage and tear turnover rates in the pre-corneal compartment drives the ocular drug bioavailability. The quick removal of drug suspended particles from the pre-corneal compartment renders the impact of particle size inconsequential relative to viscosity modification. The in vivo ocular absorption is (1) viscosity non-sensitive when the viscosity is high and the impact of viscosity on the pre-corneal residence time reaches the maximum physiological system capacity or (2) viscosity sensitive when the viscosity is below a certain limit. This study reinforces our understanding of the interplay between physiological factors and ophthalmic formulation physicochemical properties and their impact on in vivo ocular drug PK performance in rabbits.




Similar content being viewed by others
References
Center for Drug Evaluation and Research. Generic drug user fee amendments [Internet]. [cited 2018 Dec 14]. Available from: https://www.fda.gov/ForIndustry/UserFees/GenericDrugUserFees/default.htm. Accessed 30 Apr 2019.
US. FDA. Bioavailability and bioequivalence studies for orally administered drug products—general considerations [Internet]. 2003. Available from: https://www.fda.gov/media/87219/download. Accessed 30 Apr 2019.
Choi SH, Lionberger RA. Clinical, pharmacokinetic, and in vitro studies to support bioequivalence of ophthalmic drug products. AAPS J. 2016;18(4):1032–8. https://doi.org/10.1208/s12248-016-9932-z.
Orange book: approved drug products with therapeutic equivalence evaluations [Internet]. [cited 2018 Dec 14]. Available from: https://www.accessdata.fda.gov/scripts/cder/ob/. Accessed 30 Apr 2019.
Ali Y, Lehmussaari K. Industrial perspective in ocular drug delivery. Adv Drug Deliv Rev. 2006 Nov 15;58(11):1258–68.
US. FDA. Draft Guidance on Dexamethasone; Tobramycin [Internet]. 2016. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/psg/Dexamethasone Tobramycin_ophthalmic suspension NDA 50818 RV Feb 2019.pdf.. Accessed 30 Apr 2019.
US. FDA. Draft guidance on Nepafenac [Internet]. 2016. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/psg/Nepafenac_ophthalmic suspension 0.1_RLD 0021862_RV12-16.pdf. Accessed 30 Apr 2019.
US. FDA. Draft guidance on Loteprednol Etabonate [Internet]. 2018. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/psg/Loteprednol Etabonate_draft_Ophthalmic drops susp_RLD 020583_RCO2-18.pdf. Accessed 30 Apr 2019.
Sager JE, Yu J, Ragueneau-Majlessi I, Isoherranen N. Physiologically based pharmacokinetic (PBPK) modeling and simulation approaches: a systematic Review of published models, applications, and model verification. Drug Metab Dispos. 2015;43(11):1823–37.
Sieg JW, Robinson JR. Mechanistic studies on transcorneal permeation of pilocarpine. J Pharm Sci. 1976;65(12):1816–22.
Himmelstein KJ, Guvenir I, Patton TF. Preliminary pharmacokinetic model of pilocarpine uptake and distribution in the eye. J Pharm Sci. 1978;67(5):603–6.
Hui HW, Robinson JR. Effect of particle dissolution rate on ocular drug bioavailability. J Pharm Sci. 1986;75(3):280–7.
Worakul N, Robinson JR. Ocular pharmacokinetics/pharmacodynamics. Eur J Pharm Biopharm. 1997;44(1):71–83.
Deng F, Ranta V-P, Kidron H, Urtti A. General pharmacokinetic model for topically administered ocular drug dosage forms. Pharm Res. 2016;33(11):2680–90. https://doi.org/10.1007/s11095-016-1993-2.
del Amo EM, Vellonen K-S, Kidron H, Urtti A. Intravitreal clearance and volume of distribution of compounds in rabbits: in silico prediction and pharmacokinetic simulations for drug development. Eur J Pharm Biopharm. 2015;95(Pt B):215–26.
Lamminsalo M, Taskinen E, Karvinen T, Subrizi A, Murtomäki L, Urtti A, et al. Extended pharmacokinetic model of the rabbit eye for intravitreal and intracameral injections of macromolecules: quantitative analysis of anterior and posterior elimination pathways. Pharm Res. 2018;35(8):153.
Hutton-Smith LA, Gaffney EA, Byrne HM, Maini PK, Gadkar K, Mazer NA. Ocular pharmacokinetics of therapeutic antibodies given by intravitreal injection: estimation of retinal permeabilities using a 3-compartment semi-mechanistic model. Mol Pharm. 2017;14(8):2690–6. https://doi.org/10.1021/acs.molpharmaceut.7b00164.
Rimpelä A-K, Reinisalo M, Hellinen L, Grazhdankin E, Kidron H, Urtti A, et al. Implications of melanin binding in ocular drug delivery. Adv Drug Deliv Rev. 2018;126:23–43.
Grass GM, Lee VH. A model to predict aqueous humor and plasma pharmacokinetics of ocularly applied drugs. Invest Ophthalmol Vis Sci. 1993;34(7):2251–9.
Walenga RL, Babiskin AH, Zhang X, Absar M, Zhao L, Lionberger RA. Impact of vehicle physicochemical properties on modeling-based predictions of cyclosporine ophthalmic emulsion bioavailability and tear film breakup time. J Pharm Sci. 2019;108(1):620–9.
US. FDA. MAXIDEX®- dexamethasone ophthalmic suspension label [Internet]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/013422s045lbl.pdf. Accessed 30 Apr 2019.
US. FDA. TOBRADEX® (tobramycin and dexamethasone ophthalmic suspension) label [Internet]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2003/50592slr032_tobradex_lbl.pdf. Accessed 30 Apr 2019.
US. FDA. TOBRADEX® ST (tobramycin / dexamethasone ophthalmic suspension) label [Internet]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/050818lbl.pdf. Accessed 30 Apr 2019.
Chockalingam A, Xu L, Stewart S, Le Merdy M, Tsakalozou E, Fan J, et al. Protocol for evaluation of topical ophthalmic drug products in different compartments of fresh eye tissues in a rabbit model. J Pharmacol Toxicol Methods [Internet] 2018. [cited 2018 Dec 17]; Available from: http://www.sciencedirect.com/science/article/pii/S105687191830697X.
Matta MK, Narayanasamy S, Thomas CD, Xu L, Stewart S, Chockalingam A, et al. A sensitive UPLC-APCI-MS/MS method for the determination of dexamethasone and its application in an ocular tissue distribution study in rabbits following topical administration. Anal Methods. 2018 May 24;10(20):2307–16.
Dexamethasone [Internet]. [cited 2018 Dec 14]. Available from: https://www.drugbank.ca/drugs/DB01234. Accessed 30 Apr 2019.
Obach RS. Prediction of human clearance of twenty-nine drugs from hepatic microsomal intrinsic clearance data: an examination of in vitro half-life approach and nonspecific binding to microsomes. Drug Metab Dispos Biol Fate Chem. 1999;27(11):1350–9.
Yamashita S, Furubayashi T, Kataoka M, Sakane T, Sezaki H, Tokuda H. Optimized conditions for prediction of intestinal drug permeability using Caco-2 cells. Eur J Pharm Sci. 2000;10(3):195–204.
Barry BW, El Eini DI. Solubilization of hydrocortisone, dexamethasone, testosterone and progesterone by long-chain polyoxyethylene surfactants. J Pharm Pharmacol. 1976;28(3):210–8.
Choi Stephanie, Wu Yang, Kozak Darby, Zheng J. Physicochemical characterization of Tobradex and Tobradex ST under physiological conditions. Baltimore; 2017.
Prausnitz MR, Noonan JS. Permeability of cornea, sclera, and conjunctiva: a literature analysis for drug delivery to the eye. J Pharm Sci. 1998;87(12):1479–88.
Hahne M, Zorn-Kruppa M, Guzman G, Brandner JM, Haltner-Ukomado E, Wätzig H, et al. Prevalidation of a human cornea construct as an alternative to animal corneas for in vitro drug absorption studies. J Pharm Sci. 2012;101(8):2976–88. https://doi.org/10.1002/jps.23190.
Patton TF, Robinson JR. Ocular evaluation of polyvinyl alcohol vehicle in rabbits. J Pharm Sci. 1975;64(8):1312–6.
Hosseini K, Matsushima D, Johnson J, Widera G, Nyam K, Kim L, et al. Pharmacokinetic study of dexamethasone disodium phosphate using intravitreal, subconjunctival, and intravenous delivery routes in rabbits. J Ocul Pharmacol Ther. 2008;24(3):301–8.
Schoenwald RD, Stewart P. Effect of particle size on ophthalmic bioavailability of dexamethasone suspensions in rabbits. J Pharm Sci. 1980;69(4):391–4.
Lu AT, Frisella ME, Johnson KC. Dissolution modeling: factors affecting the dissolution rates of polydisperse powders. Pharm Res. 1993;10(9):1308–14.
SimulationPlus. GastroPlus 9.6 manual. 2018. https://doi.org/10.17226/25305.
TGA. Australian Public Assessment report for dexamethasone [Internet]. 2016. Available from: https://www.tga.gov.au/sites/default/files/auspar-dexamethasone-161025.docx. Accessed 30 Apr 2019.
US. FDA. Pharmacology Review(s) NDA 50-818 [Internet]. 2009. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/050818s000pharmr.pdf. Accessed 30 Apr 2019.
Makoid MC, Robinson JR. Pharmacokinetics of topically applied pilocarpine in the albino rabbit eye. J Pharm Sci. 1979;68(4):435–43.
Farkouh A, Frigo P, Czejka M. Systemic side effects of eye drops: a pharmacokinetic perspective. Clin Ophthalmol. 2016;10:2433–41.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclaimer
This article reflects the views of the authors and should not be construed to represent FDA’s views or policies.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Le Merdy, M., Fan, J., Bolger, M.B. et al. Application of Mechanistic Ocular Absorption Modeling and Simulation to Understand the Impact of Formulation Properties on Ophthalmic Bioavailability in Rabbits: a Case Study Using Dexamethasone Suspension. AAPS J 21, 65 (2019). https://doi.org/10.1208/s12248-019-0334-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-019-0334-x