Abstract
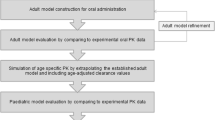
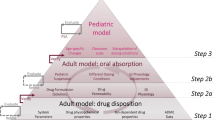
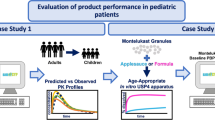
Prediction of the pharmacokinetics of orally administered drugs in children is of importance to optimize the efficacy and safety of pediatric medicines. Physiologically based pharmacokinetic (PBPK) models can be helpful for this purpose. However, application of these tools is limited by significant knowledge gaps regarding the physiological and anatomical changes which occur with age. This study aimed at investigating the age-dependent differences in oral absorption of a poorly soluble model compound, carbamazepine (CBZ) in children, infants, and neonates. We developed an oral absorption model in GastroPlus® and, after evaluation of the PBPK model for adults, extrapolation to younger ages was verified with clinical data and sensitivity analyses were applied for uncertain model parameters. We found that age-based scaling of physiological parameters, with clearance in particular, was important to obtain adequate simulation results. The sensitivity analysis revealed that CBZ absorption was influenced by solubility, particle radius, and small intestinal transit time depending on the pediatric age group and CBZ dose. However, in vitro dissolution testing using proposed pediatric biorelevant media suggested no major age dependency of dissolution kinetics. Such better understanding of oral absorption in pediatric patients is required to improve the prediction of exposure in children and the confidence in oral biopharmaceutical tools.






Similar content being viewed by others
References
Best Pharmaceuticals for Children Act, January 4, 2002 (Public Law No. 107–109).
Pediatric Research Equity Act, December 3, 2003 (Public Law No. 108–155).
Batchelor HK, Fotaki N, Klein S. Paediatric oral biopharmaceutics: key considerations and current challenges. Adv Drug Deliv Rev. 2014;73:102–26.
Batchelor HK, Kendall R, Desset-Brethes S, Alex R, Ernest TB. Application of in vitro biopharmaceutical methods in development of immediate release oral dosage forms intended for paediatric patients. Eur J Pharm Biopharm. 2013;85:833–42.
Frattarelli DA, Galinkin JL, Green TP, Johnson TD, Neville KA, Paul IM, et al. American Academy of Pediatrics Committee on Drugs. Off-label use of drugs in children. Pediatrics. 2014;133(3):563–7.
Jamei M. Recent advances in development and application of physiologically-based pharmacokinetic (PBPK) models: a transition from academic curiosity to regulatory acceptance. Curr Pharmacol Rep. 2016;2:161–9.
Leong R, Vieira ML, Zhao P, Mulugeta Y, Lee CS, Huang SM, et al. Regulatory experience with physiologically based pharmacokinetic modeling for pediatric drug trials. Clin Pharmacol Ther. 2012;91(5):926–31.
Grillo JA. Pediatric applications of PBPK modeling and simulation in drug regulatory science: where are we now? AAPS Annual Meeting and Exposition. San Diego, USA; 2014.
Batchelor H, Ernest T, Flanagan T, Klein S, Turner R, Fotaki N, et al. Towards the development of a paediatric biopharmaceutics classification system: results of a survey of experts. Int J Pharm. 2016;511(2):1151–7.
Abdel-Rahman SM, Amidon GL, Kaul A, Lukacova V, Vinks AA, Knipp GT. Summary of the National Institute of Child Health and Human Development-best pharmaceuticals for Children Act Pediatric Formulation Initiatives Workshop–Pediatric Biopharmaceutics Classification System Working Group. Clin Ther. 2012;34:S11–23.
Villiger A, Stillhart C, Parrott N, Kuentz M. Using physiologically based pharmacokinetic (PBPK) modelling to gain insights into the effect of physiological factors on oral absorption in paediatric populations. AAPS J. 2016;18(4):933–47.
Jones HM, Chen Y, Gibson C, Heimbach T, Parrott N, Peters SA, et al. Physiologically based pharmacokinetic modeling in drug discovery and development: a pharmaceutical industry perspective. Clin Pharmacol Ther. 2015;97(3):247–62.
Cristofoletti R, Charoo NA, Dressman JB. Exploratory investigation of the limiting steps of oral absorption of fluconazole and ketoconazole in children using an in silico pediatric absorption model. J Pharm Sci. 2016;105(9):2794–803.
Maharaj AR, Edginton AN, Fotaki N. Assessment of age-related changes in pediatric gastrointestinal solubility. Pharm Res. 2016;33(1):52–71.
Kerr BM, Thummel KE, Wurden CJ, Klein SM, Kroetz DL, Gonzalez FJ, et al. Human liver carbamazepine metabolism. Role of CYP3A4 and CYP2C8 in 10,11-epoxide formation. Biochem Pharmacol. 1994;47(11):1969–79.
Bertilsson L, Tomson T. Clinical pharmacokinetics and pharmacological effects of carbamazepine and carbamazepine-10,11-epoxide. An update. Clin Pharmacokinet. 1986;11(3):177–98.
Tulloch JK, Carr RR, Ensom MH. A systematic review of the pharmacokinetics of antiepileptic drugs in neonates with refractory seizures. J Pediatr Pharmacol Ther. 2012;17(1):31–44.
Patsalos PN. Carbamazepine. In: Patsalos PN, editor. Antiepileptic drug interactions. A clinical guide. 2nd ed. London: Springer; 2013. p. 11–21.
Rawlins MD, Collste P, Bertilsson L, Palmér L. Distribution and elimination kinetics of carbamazepine in man. Eur J Clin Pharmacol. 1975;8(2):91–6.
Gérardin AP, Abadie FV, Campestrini JA, Theobald W. Pharmacokinetics of carbamazepine in normal humans after single and repeated oral doses. J Pharmacol Biopharm. 1976;4(6):521–35.
Anttila M, Kahela P, Panelius M, Yrjänö T, Tikkanen R, Aaltonen R. Comparative bioavailability of two commercial preparations of carbamazepine tablets. Eur J Clin Pharmacol. 1979;15:421–5.
De Aurajo Ferreira AA, Coelho Guerra GB, da Silva SG, Dibildox E, Perez-Urizar J, et al. Comparative bioavailability of two extemporaneous solid formulations of carbamazepine against the innovator in Mexican healthy subjects. J Bioequiv Availab. 2014;6:2.
Meyer MC, Straughn AB, Jarvi EJ, Wood GC, Pelsor FR, Shah VP. The bioinequivalence of carbamazepine tablets with a history of clinical failures. Pharm Res. 1992;9(12):1612–6.
Revankar SN, Desai ND, Bhatt AD, Bolar HV, Sane SP, Gupta C, et al. Comparison of absorption rate and bioavailability of two brands of carbamazepine. J Assoc Physicians India. 1999;47(7):699–702.
Kayali A, Tuğlular I, Ertaş M. Pharmacokinetics of carbamazepine. Part I: a new bioequivalency parameter based on a relative bioavailability trial. Eur J Drug Metab Pharmacokinet. 1994;19(4):319–25.
Strandjord RE, Johannessen SI. A preliminary study of serum carbamazepine levels in healthy subjects and in patients with epilepsy. Clin Pharmacol Ther. 1975:181–8.
Morselli PL, Gerna M, de Maio D, Zanda G, Viani F, Garattini S. Pharmacokinetic studies on carbamazepine in volunteers and epileptic patients. Clin Pharmacol Anti-Epileptic Drugs. 1975:166–80.
Chan KK, Sawchuk RJ, Thompson TA, Redalieu E, Wagner WE Jr, LeSher AR, et al. Bioequivalence of carbamazepine chewable and conventional tablets: single-dose and steady-state studies. J Pharm Sci. 1985;74(8):866–70.
Kovacević I, Parojcić J, Homsek I, Tubić-Grozdanis M, Langguth P. Justification of biowaiver for carbamazepine, a low soluble high permeable compound, in solid dosage forms based on IVIVC and gastrointestinal simulation. Mol Pharm. 2009;6(1):40–7.
Cotter LM, Eadie MJ, Hooper WD, Lander CM, Smith GA, Tyrer JH. The pharmacokinetics of carbamazepine. Eur J Clin Pharmacol. 1977;12(6):451–6.
Pynnönen S, Mäntylä R, Iisalo E. Bioavailability of four different pharmaceutical preparations of carbamazepine. Acta Pharmacol Toxicol (Copenh). 1978;43(4):306–10.
Olling M, Mensinga TT, Barends DM, Groen C, Lake OA, Meulenbelt J. Bioavailability of carbamazepine from four different products and the occurrence of side effects. Biopharm Drug Dispos. 1999;20(1):19–28.
Bhatia SC, Bhatt AD, Bakshi RJ, Revankar SN, Bharucha ED, Doshi KJ, et al. Comparative bioavailability with two brands of carbamazepine-Tegretol and Mazetol in healthy volunteers. J Assoc Physicians India. 1988;36(10):611–2.
Neuvonen PJ. Bioavailability and central side effects of different carbamazepine tablets. Int J Clin Pharmacol Ther Toxicol. 1985;23(4):226–32.
Wong YY, Ludden TM, Bell RD. Effect of erythromycin on carbamazepine kinetics. Clin Pharmacol Ther. 1983;33(4):460–4.
Zhang X, Lionberger RA, Davit BM, Yu LX. Utility of physiologically based absorption modeling in implementing quality by design in drug development. AAPS J. 2011;13(1):59–71.
Levy RH, Pitlick WH, Troupin AS, Green JR, Neal JM. Pharmacokinetics of carbamazepine in normal man. Clin Pharmacol Ther. 1975;17(6):657–68.
Johannessen SI, Henriksen O, Munthe-Kaas AW, Salvesen B. Serum concentration profile studies of tablets and suppositories of valproate and carbamazepine in healthy subjects and patients with epilepsy. In: Levy RH, Pitlick WH, Eichelbaum M, Meijer J, editors. Metabolism of antiepileptic drugs. New York: Raven Press; 1984. p. 61–71.
Dam M, Christiansen J, Kristensen CB, Helles A, Jaegerskou A, Schmiegelow M. Carbamazepine: a clinical biopharmaceutical study. Eur J Clin Pharmacol. 1981;20(1):59–64.
Cotter LM, Smith G, Hooper WD, Tyrer JH, Eadie MJ. The bioavailability of carbamazepine. Proc Aust Assoc Neurol. 1975;12:123–8.
Maas B, Garnett WR, Pellock JM, Comstock TJ. A comparative bioavailability study of carbamazepine tablets and a chewable tablet formulation. Ther Drug Monit. 1987;9(1):28–33.
Sumi M, Watari N, Umezawa O, Kaneniwa N. Pharmacokinetic study of carbamazepine and its epoxide metabolite in humans. Aust J Pharm. 1987;10(11):652–61.
Kaneniwa N, Umezawa O, Watari N, Kawakami K, Asami H, Sumi M. Bioavailability and dissolution test of commercial carbamazepine tablets. Yakugaku Zasshi 1984;104(1):83–90. Japanese.
Paxton JW, Donald RA. Concentrations and kinetics of carbamazepine in whole saliva, parotid saliva, serum ultrafiltrate, and serum. Clin Pharmacol Ther. 1980;28(5):695–702.
Riad LE, Chan KK, Wagner WE Jr, Sawchuk RJ. Simultaneous first- and zero-order absorption of carbamazepine tablets in humans. J Pharm Sci. 1986;75(9):897–900.
Jung H, Milán RC, Girard ME, Montoya MA. Bioequivalence study of carbamazepine tablets: in vitro/in vivo correlation. Int J Pharm. 1997;152(1):37–44.
Neuvonen PJ, Tokola O. Bioavailability of rectally administered carbamazepine mixture. Br J Clin Pharmacol. 1987;24(6):839–41.
Hooper WD, King AR, Patterson M, Dickinson RG, Eadie MJ. Simultaneous plasma carbamazepine and carbamazepine-epoxide concentrations in pharmacokinetic and bioavailability studies. Ther Drug Monit. 1985;7(1):36–40.
Wada JA, Troupin AS, Friel P, Remick R, Leal K, Pearmain J. Pharmacokinetic comparison of tablet and suspension dosage forms of carbamazepine. Epilepsia. 1978;19(3):251–5.
Graves NM, Kriel RL, Jones-Saete C, Cloyd JC. Relative bioavailability of rectally administered carbamazepine suspension in humans. Epilepsia. 1985;26(5):429–33.
Popović J, Mikov M, Jakovljevic V. Pharmacokinetics of carbamazepine derived from a new tablet formulation. Eur J Drug Metab Pharmacokinet. 1995;20(4):297–300.
Kauko K, Tammisto P. Comparison of two generically equivalent carbamazepine preparations. Ann Clin Res. 1974;6(Suppl 11):21–5.
Bano G, Raina RK, Sharma DB. Pharmacokinetics of carbamazepine in protein energy malnutrition. Pharmacology. 1986;32(4):232–6.
Rey E, d'Athis P, de Lauture D, Dulac O, Aicardi J, Olive G. Pharmacokinetics of carbamazepine in the neonate and in the child. Int J Clin Pharmacol Biopharm. 1979;17(2):90–6.
MacKintosh DA, Baird-Lampert J, Buchanan N. Is carbamazepine an alternative maintenance therapy for neonatal seizures? Dev Pharmacol Ther. 1987;10(2):100–6.
Miles MV, Lawless ST, Tennison MB, Zaritsky AL, Greenwood RS. Rapid loading of critically ill patients with carbamazepine suspension. Pediatrics. 1990;86(2):263–6.
European Medicines Agency. ICH Topic E 11: Clinical investigation of medicinal products in the paediatric population. CPMP/ICH/2711/99. London; 2001.
Simulations Plus, Inc. GastroPlus version 9.0: simulation software for drug discovery and development (user manual). 2015; Lancaster, CA.
Parrott N, Lave T. Applications of physiologically based absorption models in drug discovery and development. Mol Pharm. 2008;5(5):760–75.
Heikkinen AT, Baneyx G, Caruso A, Parrott N. Application of PBPK modeling to predict human intestinal metabolism of CYP3A substrates—an evaluation and case study using GastroPlus. Eur J Pharm Sci. 2012;47(2):375–86.
Jones H, Parrott N, Ohlenbusch G, Lavé T. Predicting pharmacokinetic food effects using biorelevant solubility media and physiologically based modelling. Clin Pharm. 2006;45(12):1213–26.
Parrott N, Lave T. Computer models for predicting drug absorption. In: Dressman J, Reppas C, editors. Oral drug absorption—prediction and assessment. Boca Raton: CRC Press; 2010. p. 338–55.
Jorga K, Chavanne C, Frey N, Lave T, Lukacova V, Parrott N, et al. Bottom-up meets top-down: complementary physiologically based pharmacokinetic and population pharmacokinetic modeling for regulatory approval of a dosing algorithm of valganciclovir in very young children. Clin Pharmacol Ther. 2016;100(6):761–9.
Lukacova V, Goelzer P, Reddy M, Greig G, Reigner B, Parrott N. A physiologically based pharmacokinetic model for ganciclovir and its prodrug valganciclovir in adults and children. AAPS J. 2016;18(6):1453–63.
Haddad S, Restieri C, Krishnan K. Characterization of age-related changes in body weight and organ weights from birth to adolescence in humans. J Toxicol Environ Health A. 2001;64(6):453–64.
Johnson TN, Tucker GT, Tanner MS, Rostami-Hodjegan A. Changes in liver volume from birth to adulthood: a meta-analysis. Liver Transpl. 2005;11(12):1481–93.
McNamara PJ, Alcorn J. Protein binding predictions in infants. AAPS PharmSciTech. 2002;4(1):E4.
Rubin MI, Bruck E, Rapport M. Maturation of renal function in childhood: clearance studies. J Clin Invest. 1949;28(5 Pt 2):1144–62.
Yanowitz TD, Yao AC, Pettigrew KD, Werner JC, Oh W, Stonestreet BS. Postnatal hemodynamic changes in very-low-birth weight infants. J Appl Physiol. 1999;87(1):370–80.
Johnson TN, Rostami-Hodjegan A, Tucker GT. Prediction of the clearance of eleven drugs and associated variability in neonates, infants and children. Clin Pharmacokinet. 2006;45:931–56.
Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP. Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health. 1997;13(4):407–84.
LL de Zwart, CJM Rompelberg, AJAM Sips, Welink J, JGM van Engelen. Anatomical and physiological differences between various species used in studies on the pharmacokinetics and toxicology of xenobiotics. A review of literature. Bilthoven (NL): National Institute of Public Health and the Environment; 1999 Report No.: 623860 010.
Van Den Driessche M, Van Malderen N, Geypens B, Ghoos Y, Veereman-Wauters G. Lactose-[13C]ureide breath test: a new, noninvasive technique to determine orocecal transit time in children. J Pediatr Gastroenterol Nutr. 2000;31(4):433–8.
Zhang SC, Wang WL, Bai YZ, Yuan ZW, Wang W. Determination of total and segmental colonic transit time in constipated children. Zhonghua Er Ke Za Zhi 2003;41(3):176–179. Chinese.
Bautista Casasnovas A, Varela Cives R, Villanueva Jeremias A, Castro-Gago M, Cadranel S, Tojo SR. Measurement of colonic transit time in children. J Pediatr Gastroenterol Nutr. 1999;13(1):42–5.
Rodgers T, Rowland M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci. 2006;95(6):1238–57.
European Medicines Agency; Committee for Medicinal Products for Human Use (CHMP). Reflection paper: formulations of choice for the paediatric population. EMEA/CHMP/PEG194810/2005. London; 2006.
Reith DM, Hooper WD, Parke J, Charles B. Population pharmacokinetic modeling of steady state carbamazepine clearance in children, adolescents, and adults. J Pharmacokinet Pharmacodyn. 2001;28(1):79–92.
Delgado Iribarnegaray MF, Santo Bueldga D, García Sánchez MJ, Otero MJ, Falcão AC, Domínguez-Gil A. Carbamazepine population pharmacokinetics in children: mixed-effect models. Ther Drug Monit. 1997;19(2):132–9.
Yukawa E, Aoyama T. Detection of carbamazepine drug interaction by multiple peak approach screening using routine clinical pharmacokinetic data. J Clin Pharmacol. 1996;36(8):752–9.
Gray AL, Botha JH, Miller R. A model for the determination of carbamazepine clearance in children on mono- and polytherapy. Eur J Clin Pharmacol. 1998;54(4):359–62.
Table for body surface area—body weight correlation in children. [Internet] National Institute for Health and Care Excellence Great Britain [cited 21 Sep 2016]. Available from: https://www.evidence.nhs.uk/formulary/bnfc/current/body-surface-area-in-children.
Galia E, Nicolaides E, Hörter D, Löbenberg R, Reppas C, Dressman JB. Evaluation of various dissolution media for predicting in vivo performance of class I and II drugs. Pharm Res. 1998;15(5):698–705.
biorelevant.com [Internet]. London: Instructions for preparation of FaSSIF, FeSSIF, and FaSSGF [cited 3 June 2017]. Available from: https://biorelevant.com/fassif-fessif-fassgf/how-to-make/.
Upreti VV, Wahlstrom JL. Meta-analysis of hepatic cytochrome P450 ontogeny to underwrite the prediction of pediatric pharmacokinetics using physiologically based pharmacokinetic modeling. J Clin Pharmacol. 2015;2(10):585.
Magnusson M, et al. Pharmacodynamics of carbamazepine-mediated induction of CYP3A4, CYP1A2, and Pgp as assessed by probe substrates midazolam, caffeine, and digoxin. Clin Pharmacol Ther. 2008;84(1)
Bertilsson L, et al. Autoinduction of carbamazepine metabolism in children examined by a stable isotope technique. Clin Pharmacol Ther. 1980;27(1):83–8.
Biochemical pharmacology Volume 47, Issue 11, 1 June 1994, Pages 1969–1979.
Wang P, et al. Effects of CYP3A4/5 and ABCB1 genetic polymorphisms on carbamazepine metabolism and transport in Chinese patients with epilepsy treated with carbamazepine in monotherapy and bitherapy. Epilepsy Res. 2015;117:52–7.
Gandhi SV, Rodriguez W, Khan M, Polli JE. Considerations for a Pediatric Biopharmaceutics Classification System (BCS): application to five drugs. AAPS PharmSciTech. 2014;15(3):601–11.
Shawahna R. Pediatric biopharmaceutical classification system: using age-appropriate initial gastric volume. AAPS J. 2016;18(3):728–36.
Nicolas JM, Bouzom F, Hugues C, Ungell AL. Oral drug absorption in pediatrics: the intestinal wall, its developmental changes and current tools for predictions. Biopharm Drug Dispos. 2017;38(3):209–30.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
ESM 1
(DOCX 1580 kb)
Rights and permissions
About this article
Cite this article
Kohlmann, P., Stillhart, C., Kuentz, M. et al. Investigating Oral Absorption of Carbamazepine in Pediatric Populations. AAPS J 19, 1864–1877 (2017). https://doi.org/10.1208/s12248-017-0149-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-017-0149-6