Abstract
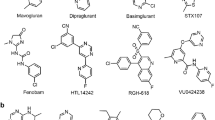
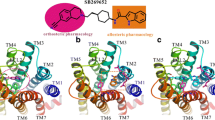
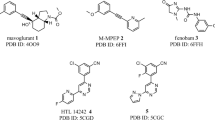
GPCR allosteric modulators target at the allosteric binding pockets of G protein-coupled receptors (GPCRs) with indirect influence on the effects of an orthosteric ligand. Such modulators exhibit significant advantages compared to the corresponding orthosteric ligands, including better chemical tractability or physicochemical properties, improved selectivity, and reduced risk of oversensitization towards their receptors. Metabotropic glutamate receptor 5 (mGlu5), a member of class C GPCRs, is a promising therapeutic target for treating many central nervous system diseases. The crystal structure of mGlu5 in the complex with the negative allosteric modulator mavoglurant was recently reported, providing a fundamental model for designing new allosteric modulators. Computational fragment-based drug discovery represents a powerful scaffold-hopping and lead structure-optimization tool for drug design. In the present work, a set of integrated computational methodologies was first used, such as fragment library generation and retrosynthetic combinatorial analysis procedure (RECAP) for novel compound generation. Then, the compounds generated were assessed by benchmark dataset verification, docking studies, and QSAR model simulation. Subsequently, structurally diverse compounds, with reported or unreported scaffolds, can be observed from top 20 in silico synthesized compounds, which were predicted to be potential mGlu5 modulators. In silico compounds with reported scaffolds may fill SAR holes in known, patented series of mGlu5 modulators. And the generation of compounds without reported tests on mGluR indicates that our approach is doable for exploring and designing novel compounds. Our case study of designing allosteric modulators on mGlu5 demonstrated that the established computational fragment-based approach is a useful methodology for facilitating new compound design in the future.








Similar content being viewed by others
References
Wu H, Wang C, Gregory KJ, Han GW, Cho HP, Xia Y, et al. Structure of a class C GPCR metabotropic glutamate receptor 1 bound to an allosteric modulator. Science. 2014;344(6179):58–64.
Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, et al. The sequence of the human genome. Science. 2001;291(5507):1304–51.
Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5(12):993–6.
Feng Z, Hu G, Ma S, Xie X-Q. Computational advances for the development of allosteric modulators and bitopic ligands in G protein-coupled receptors. AAPS J. 2015;17(5):1080–95.
Wenthur CJ, Gentry PR, Mathews TP, Lindsley CW. Drugs for allosteric sites on receptors. Annu Rev Pharmacol Toxicol. 2014;54:165.
Wellendorph P, Bräuner-Osborne H. Molecular basis for amino acid sensing by family CG-protein-coupled receptors. Br J Pharmacol. 2009;156(6):869–84.
Lane JR, Abdul-Ridha A, Canals M. Regulation of G protein-coupled receptors by allosteric ligands. ACS Chem Neurosci. 2013;4(4):527–34.
Melancon BJ, Hopkins CR, Wood MR, Emmitte KA, Niswender CM, Christopoulos A, et al. Allosteric modulation of seven transmembrane spanning receptors: theory, practice, and opportunities for central nervous system drug discovery. J Med Chem. 2012;55(4):1445–64.
Christopoulos A. Allosteric binding sites on cell-surface receptors: novel targets for drug discovery. Nat Rev Drug Discov. 2002;1(3):198–210.
Conn PJ, Christopoulos A, Lindsley CW. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Discov. 2009;8(1):41–54.
Bridges TM, Lindsley CW. G-protein-coupled receptors: from classical modes of modulation to allosteric mechanisms. ACS Chem Biol. 2008;3(9):530–41.
Digby GJ, Conn PJ, Lindsley CW. Orthosteric-and allosteric-induced ligand-directed trafficking at GPCRs. Curr Opin Drug Discov Devel. 2010;13(5):587.
Fang Z, Grütter C, Rauh D. Strategies for the selective regulation of kinases with allosteric modulators: exploiting exclusive structural features. ACS Chem Biol. 2012;8(1):58–70.
Möhler H, Fritschy J, Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002;300(1):2–8.
Pin J-P, Galvez T, Prézeau L. Evolution, structure, and activation mechanism of family 3/C G-protein-coupled receptors. Pharmacol Ther. 2003;98(3):325–54.
Doré AS, Okrasa K, Patel JC, Serrano-Vega M, Bennett K, Cooke RM, et al. Structure of class C GPCR metabotropic glutamate receptor 5 transmembrane domain. Nature. 2014;511(7511):557–62.
Li G, Jørgensen M, Campbell BM. Metabotropic glutamate receptor 5-negative allosteric modulators for the treatment of psychiatric and neurological disorders (2009-July 2013). Pharmaceutical patent analyst. 2013;2(6):767–802.
Levenga J, Hayashi S, de Vrij FM, Koekkoek SK, van der Linde HC, Nieuwenhuizen I, et al. AFQ056, a new mGluR5 antagonist for treatment of fragile X syndrome. Neurobiol Dis. 2011;42(3):311–7.
Ritzén A, Sindet R, Hentzer M, Svendsen N, Brodbeck RM, Bundgaard C. Discovery of a potent and brain penetrant mGluR5 positive allosteric modulator. Bioorg Med Chem Lett. 2009;19(12):3275–8.
Hajduk PJ, Greer J. A decade of fragment-based drug design: strategic advances and lessons learned. Nat Rev Drug Discov. 2007;6(3):211–9.
Joseph-McCarthy D, Campbell AJ, Kern G, Moustakas D. Fragment-based lead discovery and design. J Chem Inf Model. 2014;54(3):693–704.
Allen KN, Bellamacina CR, Ding X, Jeffery CJ, Mattos C, Petsko GA, et al. An experimental approach to mapping the binding surfaces of crystalline proteins. J Phys Chem. 1996;100(7):2605–11.
Chessari G, Woodhead AJ. From fragment to clinical candidate—a historical perspective. Drug Discov Today. 2009;14(13):668–75.
de Kloe GE, Bailey D, Leurs R, de Esch IJ. Transforming fragments into candidates: small becomes big in medicinal chemistry. Drug Discov Today. 2009;14(13):630–46.
Jencks WP. On the attribution and additivity of binding energies. Proc Natl Acad Sci. 1981;78(7):4046–50.
Schneider G, Fechner U. Computer-based de novo design of drug-like molecules. Nat Rev Drug Discov. 2005;4(8):649–63.
Howard N, Abell C, Blakemore W, Chessari G, Congreve M, Howard S, et al. Application of fragment screening and fragment linking to the discovery of novel thrombin inhibitors. J Med Chem. 2006;49(4):1346–55.
Edwards PD, Albert JS, Sylvester M, Aharony D, Andisik D, Callaghan O, et al. Application of fragment-based lead generation to the discovery of novel, cyclic Amidine β-secretase inhibitors with nanomolar potency, cellular activity, and high ligand efficiency §. J Med Chem. 2007;50(24):5912–25.
Sun C, Petros AM, Hajduk PJ. Fragment-based lead discovery: challenges and opportunities. J Comput Aided Mol Des. 2011;25(7):607–10.
Loving K, Alberts I, Sherman W. Computational approaches for fragment-based and de novo design. Curr Top Med Chem. 2010;10(1):14–32.
Schuffenhauer A, Ruedisser S, Marzinzik A, Jahnke W, Selzer P, Jacoby E. Library design for fragment based screening. Curr Top Med Chem. 2005;5(8):751–62.
Siegal G, Eiso A, Schultz J. Integration of fragment screening and library design. Drug Discov Today. 2007;12(23):1032–9.
Lepre C. Fragment-based drug discovery using the SHAPES method. Expert Opin Drug Discovery. 2007;2(12):1555–66.
Fjellström O, Akkaya S, Beisel H-G, Eriksson P-O, Erixon K, Gustafsson D, et al. Creating novel activated factor xi inhibitors through fragment based lead generation and structure aided drug design. PLoS One. 2015;10(1):e0113705.
Ni S, Yuan Y, Huang J, Mao X, Lv M, Zhu J, et al. Discovering potent small molecule inhibitors of cyclophilin A using de novo drug design approach. J Med Chem. 2009;52(17):5295–8.
Huang Z, Mou L, Shen Q, Lu S, Li C, Liu X, et al. ASD v2. 0: updated content and novel features focusing on allosteric regulation. Nucleic Acids Res. 2014;42(D1):D510–D6.
Christopher JA, Aves SJ, Bennett KA, Doré AS, Errey JC, Jazayeri A, et al. Fragment and structure-based drug discovery for a class C GPCR: discovery of the mGlu5 negative allosteric modulator HTL14242 (3-chloro-5-[6-(5-fluoropyridin-2-yl) pyrimidin-4-yl] benzonitrile). J Med Chem. 2015;58(16):6653–64.
SYBYL-X 1.3 TI, 1699 South Hanley Rd., St. Louis, MO, 63144, USA. 2010. 2010.
Feng Z, Ma S, Hu G, Xie X-Q. Allosteric binding site and activation mechanism of class C G-protein coupled receptors: metabotropic glutamate receptor family. AAPS J. 2015;17(3):737–53.
The PyMOL Molecular graphics system, version 1.5.0.1 Schrodinger, LLC.
Lewell XQ, Judd DB, Watson SP, Hann MM. RECAP Retrosynthetic Combinatorial Analysis Procedure: a powerful new technique for identifying privileged molecular fragments with useful applications in combinatorial chemistry. J Chem Inf Comput Sci. 1998;38(3):511–22.
Fechner U, Schneider G. Flux (1): a virtual synthesis scheme for fragment-based de novo design. J Chem Inf Model. 2006;46(2):699–707.
Teague SJ, Davis AM, Leeson PD, Oprea T. The design of leadlike combinatorial libraries. Angew Chem Int Ed. 1999;38(24):3743–8.
Jain AN. Scoring noncovalent protein-ligand interactions: a continuous differentiable function tuned to compute binding affinities. J Comput Aided Mol Des. 1996;10(5):427–40.
Weiner SJ, Kollman PA, Nguyen DT, Case DA. An all atom force field for simulations of proteins and nucleic acids. J Comput Chem. 1986;7(2):230–52.
Meng EC, Shoichet BK, Kuntz ID. Automated docking with grid-based energy evaluation. J Comput Chem. 1992;13(4):505–24.
Sanner MF. Python: a programming language for software integration and development. J Mol Graph Model. 1999;17(1):57–61.
Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, et al. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem. 1998;19(14):1639–62.
Huang N, Shoichet BK, Irwin JJ. Benchmarking sets for molecular docking. J Med Chem. 2006;49(23):6789–801.
Weiss DR, Bortolato A, Tehan B, Mason JS. GPCR-bench: a benchmarking set and practitioners’ guide for G protein-coupled receptor docking. J Chem Inf Model. 2016;56(4):642–51.
Mysinger MM, Carchia M, Irwin JJ, Shoichet BK. Directory of useful decoys, enhanced (DUD-E): better ligands and decoys for better benchmarking. J Med Chem. 2012;55(14):6582–94.
Ioele G, De Luca M, Oliverio F, Ragno G. Prediction of photosensitivity of 1, 4-dihydropyridine antihypertensives by quantitative structure-property relationship. Talanta. 2009;79(5):1418–24.
Raska I, Toropov A. Comparison of QSPR models of octanol/water partition coefficient for vitamins and non vitamins. Eur J Med Chem. 2006;41(11):1271–8.
Hogg R, Tanis E. Nonparametric methods. Probability and statistical inference. 4th ed. New York: MacMillan Publishing Co; 1993. p. 589–646.
Hogg RV, Tanis EA. Solutions Manual, Probability and Statistical Interference: Solutions: Macmillan; 1988.
Ertl P. Cheminformatics analysis of organic substituents: identification of the most common substituents, calculation of substituent properties, and automatic identification of drug-like bioisosteric groups. J Chem Inf Comput Sci. 2003;43(2):374–80.
Congreve M, Carr R, Murray C, Jhoti H. A ‘rule of three’ for fragment-based lead discovery? Drug Discov Today. 2003;8(19):876–7.
Rees DC, Congreve M, Murray CW, Carr R. Fragment-based lead discovery. Nat Rev Drug Discov. 2004;3(8):660–72.
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2012;64:4–17.
Bogan AA, Thorn KS. Anatomy of hot spots in protein interfaces. J Mol Biol. 1998;280(1):1–9.
Leffler JE. The enthalpy-entropy relationship and its implications for organic chemistry. The Journal of Organic Chemistry. 1955;20(9):1202–31.
Eftink MR, Anusiem A, Biltonen RL. Enthalpy-entropy compensation and heat capacity changes for protein-ligand interactions: general thermodynamic models and data for the binding of nucleotides to ribonuclease A. Biochemistry. 1983;22(16):3884–96.
Davenport AP, Russell FD. Radioligand binding assays: theory and practice. Current Directions in Radiopharmaceutical Research and Development: Springer; 1996; 169–79.
Cui W, Yan X. Adaptive weighted least square support vector machine regression integrated with outlier detection and its application in QSAR. Chemom Intell Lab Syst. 2009;98(2):130–5.
Baell JB, Holloway GA. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J Med Chem. 2010;53(7):2719–40.
Patlewicz G, Jeliazkova N, Safford R, Worth A, Aleksiev B. An evaluation of the implementation of the Cramer classification scheme in the Toxtree software. SAR QSAR Environ Res. 2008;19(5–6):495–524.
Bursavich MG, Gilbert AM, Stock JR. Piperazine metabotropic glutamate receptor 5 (MGLUR5) negative allosteric modulators for anxiety/depression. US Patent; 2009.
Macdonald G, Tresadern G, Trabanco-Suarez A, Pastor-Fernandez J. Preparation of bicyclic thiazoles as allosteric modulators of mGluR5 receptors. WO Patent; 2011.
Imogai H, Mutel V, Duvey GA, Cid-Nunez J, Le Poul E, Lutjens R. Novel thieno-pyridine and thieno-pyrimidine derivatives and their use as positive allosteric modulators of mGluR2-receptors. US Patent; 2005.
Conn J, Lindsley C, Weaver C, Stauffer S, Williams R, McDonald G, et al. Preparation of O-benzyl nicotinamide analogs as mGluR5 positive allosteric modulators. WO Patent. 2011;
Imogai HJ, Cid-Nunez JM, Andres-Gil JI, Trabanco-Suarez AA, Santamarina JO, Dautzenberg FM, et al.. 1, 4-Disubstituted 3-cyano-pyridone derivatives and their use as positive allosteric modulators of MGLUR2-receptors. US Patent; 2014.
Acknowledgements
Authors would like to acknowledge the funding supports to the Xie laboratory from the NIH NIDA (P30 DA035778A1), NIH (R01 DA025612), and DOD (W81XWH-16-1-0490).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Electronic supplementary material
Supplementary table 1
(DOCX 851 kb)
Supplementary table 2
(DOCX 548 kb)
Supplementary table 3
(DOCX 1180 kb)
Supplementary table 4
(DOCX 464 kb)
Supplementary table 5
(DOCX 555 kb)
Rights and permissions
About this article
Cite this article
Bian, Y., Feng, Z., Yang, P. et al. Integrated In Silico Fragment-Based Drug Design: Case Study with Allosteric Modulators on Metabotropic Glutamate Receptor 5. AAPS J 19, 1235–1248 (2017). https://doi.org/10.1208/s12248-017-0093-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-017-0093-5