Abstract
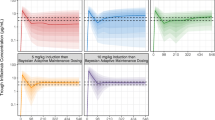
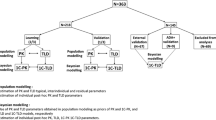
Standard of care (SOC; combination of 5–10 mg/kg and an interval every 6–8 weeks) dosing of infliximab (IFX) is associated with significant loss of response. Dashboards using covariates that influence IFX pharmacokinetics (PK) may be a more precise way of optimizing anti-TNF dosing. We tested a prototype dashboard to compare forecasted dosing regimens with actual administered regimens and SOC. Fifty IBD patients completing IFX induction were monitored during maintenance (weeks 14–54). Clinical and laboratory data were collected at each infusion; serum was analyzed for IFX concentrations and anti-drug antibodies (ADA) at weeks 14 and 54 (Prometheus Labs, San Diego). Dosing was blinded to PK data. Dashboard-based assessments were conducted on de-identified clinical, laboratory, and PK data. Bayesian algorithms were used to forecast individualized troughs and determine optimal dosing to maintain target trough concentrations (3 μg/mL). Dashboard forecasted dosing post-week 14 was compared to actual administered dose and frequency and SOC. Using week 14 clinical data only, the dashboard recommended either a dose or an interval change (<0.5 mg/kg or <1 week difference) in 43/50 patients; only 44% recommended to have SOC dosing. When IFX14 concentration and ADA status were added to clinical data, dose and/or interval changes based on actual dosing were recommended in 48/50 (96%) patients; SOC dosing was recommended in only 11/50 (22%). Dashboard recommended SOC IFX dosing in a minority of patients. Dashboards will be an important tool to individualize IFX dosing to improve treatment durability.



Similar content being viewed by others
REFERENCES
Sherman M, Tsynman DN, Kim A, et al. Sustained improvement in health-related quality of life measures in patients with inflammatory bowel disease receiving prolonged anti-tumor necrosis factor therapy. J Dig Dis. 2014;15:174–9.
Vogelaar L, Spijker AV, van der Woude CJ. The impact of biologics on health-related quality of life in patients with inflammatory bowel disease. Clin Exp Gastroenterol. 2009;2:101–9.
Lichtenstein GR, Yan S, Bala M, et al. Infliximab maintenance treatment reduces hospitalizations, surgeries, and procedures in fistulizing Crohn’s disease. Gastroenterology. 2005;128:862–8.
Peyrin-Biroulet L, Deltenre P, de Suray N, et al. Efficacy and safety of tumor necrosis factor antagonists in Crohn’s disease: meta-analysis of placebo-controlled trials. Clin Gastroenterol Hepatol. 2008;6:644–53.
Ordás I, Mould DR, Feagan BG, et al. Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin Pharmacol Ther. 2012;91:635–46.
Ordás I, Feagan BG, Sandborn WJ. Therapeutic drug monitoring of tumor necrosis factor antagonists in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2012;10:1079–87.
Yarur AJ, Abreu MT, Deshpande AR, et al. Therapeutic drug monitoring in patients with inflammatory bowel disease. World J Gastroenterol. 2014;20:3475–84.
Pan W-J, Gibbs M, Frame B, Mould DR. Model based meta analyses of disease metrics in patients with Crohn’s disease. ACCP National Meeting 2012 Chicago IL.
Maser E, Villela R, Silverberg M, et al. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn’s disease. Clin Gastroenterol Hepatol. 2006;4:1248–54.
Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med. 2003;348:601–8.
Afif W, Loftus Jr EV, Faubion WA, et al. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol. 2010;105:1133–9.
Singh N, Rosenthal CJ, Melmed GY, et al. Early infliximab trough levels are associated with persistent remission in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:1708–13.
Cornillie F, Hanauer SB, Diamond RH, et al. Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT I trial. Gut. 2014;63:1721–7.
Vaughn BP, Martinez-Vazquez M, Patwardhan VR, et al. Proactive therapeutic concentration monitoring of infliximab may improve outcomes for patients with inflammatory bowel disease: results from a pilot observational study. Inflamm Bowel Dis. 2014;20:1996–2003.
Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015;148:1320–9.
Zhao Q, Tensfeldt TG, Chandra R, et al. Population pharmacokinetics of azithromycin and chloroquine in healthy adults and pediatric malaria subjects following oral administration of fixed dose azithromycin and chloroquine combination tablets. Malar J. 2014;13:36.
Mould DR, Dubinsky MC. Dashboard systems: pharmacokinetic/pharmacodynamic mediated dose optimization. J Clin Pharmacol. 2015;55 Suppl 3:S51–9.
Montazeri A, Culine S, Laguerre B, et al. Individual adaptive dosing of topotecan in ovarian cancer. Clin Cancer Res. 2002;8:394–9.
Barrett JS, Mondick JT, Narayan M, et al. Integration of modeling and simulation into hospital-based decision support systems guiding pediatric pharmacotherapy. BMC Med Inform Decis Mak. 2008;8:6.
Mould DR, Moyer B, Amur S, et al. The impact of new technologies on the science of clinical care and drug development. AAPS Magazine December 2013.
Dubinsky MC, Mei L, Friedman M, et al. Genome wide association (GWA) predictors of anti-TNFalpha therapeutic responsiveness in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1357–66.
Mould DR. Why therapeutic drug monitoring is needed for monoclonal antibodies and how do we implement this? Clin Pharmacol Ther. 2016;99:351–4.
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; 2013. URL Http://www.R-project.org.
Mould DR, Upton RN, Wojciechowski J. Dashboard systems: implementing pharmacometrics from bench to bedside. AAPS J. 2014;16:925–37.
Xu Z, Mould DR, Hu C, Ford J, Keen M, Davis HM, et al. A population-based pharmacokinetic pooled analysis of infliximab in pediatrics. ACCP National Meeting 2012 San Diego CA.
Vande Casteele N, Khanna R, Levesque BG, et al. The relationship between infliximab concentrations, antibodies to infliximab and disease activity in Crohn’s disease. Gut. 2015;64:1539–45.
Vande Casteele N, Gils A, Singh S, et al. Antibody response to infliximab and its impact on pharmacokinetics can be transient. Am J Gastroenterol. 2013;108:962–71.
Ben-Horin S, Waterman M, Kopylov U, et al. Addition of an immunomodulator to infliximab therapy eliminates antidrug antibodies in serum and restores clinical response of patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:444–7.
Van Lent-Evers NA, Mathôt RA, Geus WP, et al. Impact of goal-oriented and model-based clinical pharmacokinetic dosing of aminoglycosides on clinical outcome: a cost-effectiveness analysis. Ther Drug Monit. 1999;21:63–73.
Author Contributions
1. Conception and design of the study: MCD and DRM
2. Generation, collection, assembly, analysis, and interpretation of data: MCD, DRM, and BLP
3. Drafting or revision of the manuscript MCD, DRM, and BLP
4. Approval of the final version of the manuscript: MCD, DRM, BLP, SR, and NS
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by CSMC Institutional Review Board (IRB no. 19251); all patients signed informed consent before enrollment.
Disclosures
Marla C. Dubinsky: Consultant for Prometheus Labs, Janssen, AbbVie
Becky L. Phan: Nothing to disclose
Namita Singh: Janssen—research grant
Shervin Rabizadeh: Consultant for Prometheus Labs
Diane R. Mould: President of Projections Research Inc. a consulting company for the Pharmaceutical Industry
Grant Support
Partially funded by the NIH/NIDDK (1R21DK084554-01) (MCD), Claire and Abe Levine Chair in Pediatric IBD Research (MCD).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
(DOCX 19 kb)
Supplementary Table 2
(DOCX 23 kb)
Supplementary Table 3
(DOCX 21 kb)
Supplementary Table 4
(DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Dubinsky, M.C., Phan, B.L., Singh, N. et al. Pharmacokinetic Dashboard-Recommended Dosing Is Different than Standard of Care Dosing in Infliximab-Treated Pediatric IBD Patients. AAPS J 19, 215–222 (2017). https://doi.org/10.1208/s12248-016-9994-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-016-9994-y