Abstract
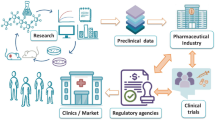
Advancing nanomedicines from concept to clinic requires integration of new science with traditional pharmaceutical development. The medical and commercial success of nanomedicines is greatly facilitated when those charged with developing nanomedicines are cognizant of the unique opportunities and technical challenges that these products present. These individuals must also be knowledgeable about the processes of clinical and product development, including regulatory considerations, to maximize the odds for successful product registration. This article outlines these topics with a goal to accelerate the combination of academic innovation with collaborative industrial scientists who understand pharmaceutical development and regulatory approval requirements—only together can they realize the full potential of nanomedicines for patients.
Similar content being viewed by others
References
Langer R, Weissleder R. Nanotechnol JAMA. 2015;313:135–6.
Finch G, Havel H, Analoui M, Barton RW, Diwan AR, Hennessy M, et al. Nanomedicine drug development: a scientific symposium entitled charting a roadmap to commercialization. AAPS J. 2014;16:1–7.
Eaton MA, Levy L, Fontaine OM. Delivering nanomedicines to patients: a practical guide. Nanomed: Nanotechnol, Biol Med. 2015;11:983–92.
Prabhakar U, Maeda H, Jain RK, Sevick-Muraca EM, Zamboni W, Farokhzad OC, et al. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013;73:2412–7.
Harrington KJ, Mohammadtaghi S, Uster PS, Glass D, Peters AM, Vile RG, et al. Effective targeting of solid tumors in patients with locally advanced cancers by radiolabeled pegylated liposomes. Clin Cancer Res. 2001;7:243–54.
Hendriks B, Shields A, Siegel BA, Miller K, Munster P, Ma C, et al. PET/CT imaging of 64Cu-labelled HER2 liposomal doxorubicin (64Cu-MM-302) quantifies variability of liposomal drug delivery to diverse tumor lesions in HER2-positive breast cancer patients. Ann Oncol. 2014;25:i19.
Ramanathan RK, Korn RL, Sachdev JC, Fetterly GJ, Marceau K, Marsh V, et al. Abstract CT224: Pilot study in patients with advanced solid tumors to evaluate feasibility of ferumoxytol (FMX) as tumor imaging agent prior to MM-398, a nanoliposomal irinotecan (nal-IRI). Cancer Res. 2014;74:CT224.
Low S, Von Hoff D, Mita M, Burris H, Eisenberg P, Hart L, et al. Prostate-specific membrane antigen (PSMA) expression as a potential patient selection marker in patients with refractory solid tumors administered BIND-014, a PSMA-targeted nanoparticle containing docetaxel. Cancer Res. 2014;74:911.
Hrkach J, Von Hoff D, Ali MM, Andrianova E, Auer J, Campbell T, et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci Transl Med. 2012;4:128ra39.
Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of pegylated liposomal doxorubicin. Clin Pharmacokinet. 2003;42:419–36.
Sparreboom A, Scripture CD, Trieu V, Williams PJ, De T, Yang A, et al. Comparative preclinical and clinical pharmacokinetics of a cremophor-free, nanoparticle albumin-bound paclitaxel (ABI-007) and paclitaxel formulated in Cremophor (Taxol). Clin Cancer Res. 2005;11:4136–43.
Krishna R, Webb MS, Onge GS, Mayer LD. Liposomal and nonliposomal drug pharmacokinetics after administration of liposome-encapsulated vincristine and their contribution to drug tissue distribution properties. J Pharmacol Exp Ther. 2001;298:1206–12.
Wei GL, Xiao SH, Gu Y, Si DY. Validated HPLC assay of liposome-encapsulated and non-liposomal doxorubicin in plasma and its application to pharmacokinetic study. J Pharm Pharmacol. 2010;10:229–36.
Ait-Oudhia S, Mager DE, Straubinger RM. Application of pharmacokinetic and pharmacodynamic analysis to the development of liposomal formulations for oncology. Pharmaceutics. 2014;6:137–74.
Drummond DC, Noble CO, Hayes ME, Park JW, Kirpotin DB. Pharmacokinetics and in vivo drug release rates in liposomal nanocarrier development. J Pharmaceutical Sci. 2008;97:4696–740.
Johnson JI, Decker S, Zaharevitz D, Rubinstein LV, Venditti JM, Schepartz S, et al. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer. 2001;84:1424.
Gould SE, Junttila MR, de Sauvage FJ. Translational value of mouse models in oncology drug development. Nat Med. 2015;21:431–9.
Caron WP, Morgan KP, Zamboni BA, Zamboni WC. A review of study designs and outcomes of phase I clinical studies of nanoparticle agents compared with small-molecule anticancer agents. Clin Cancer Res. 2013;19:3309–15.
Qiu S, Liu Z, Hou L, Li Y, Wang J, Wang H, et al. Complement activation associated with polysorbate 80 in beagle dogs. Int Immunopharmacol. 2013;15:144–9.
Masini E, Planchenault J, Pezziardi F, Gautier P, Gagnol JP. Histamine-releasing properties of polysorbate 80 in vitro and in vivo: correlation with its hypotensive action in the dog. Agents Actions. 1985;16:470–7.
Dobrovolskaia MA, McNeil SE. Understanding the correlation between in vitro and in vivo immunotoxicity tests for nanomedicines. J Control Release. 2013;172:456–66.
Casinghino S, Gauthier L, McClintock D, Boldenow E, Nauman C, Freeman GB, et al. In vitro methods to predict immune-mediated toxicities of dextran-based nanomaterial precursors in rats. Toxicologist. 2009;108:178.
Cruz CN, Tyner KM, Velazquez L, Hyams KC, Jacobs A, Shaw AB, et al. CDER risk assessment exercise to evaluate potential risks from the use of nanomaterials in drug products. AAPS J. 2013;15:1–6.
Ashton S, Song YH, Nolan J, Cadogan E, Murray J, Odedra R, et al. Aurora kinase inhibitor nanoparticles target tumors with favorable therapeutic index in vivo. Sci Transl Med. 2016;8:325ra17.
Svenson S, Wolfgang M, Hwang J, Ryan J, Eliasof S. Preclinical to clinical development of the novel camptothecin nanopharmaceutical CRLX101. J Control Release. 2011;153:49–55.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Katherine Tyner, Sau (Larry) Lee, and Marc Wolfgang
Rights and permissions
About this article
Cite this article
Havel, H., Finch, G., Strode, P. et al. Nanomedicines: From Bench to Bedside and Beyond. AAPS J 18, 1373–1378 (2016). https://doi.org/10.1208/s12248-016-9961-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-016-9961-7