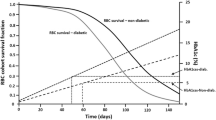
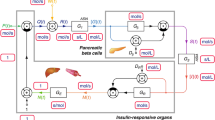
Abstract
Studying the critical transitional phase between healthy to overtly diabetic in type 2 diabetes mellitus (T2DM) is of interest, but acquiring such clinical data is impractical due to ethical concerns and would require a long study duration. A population model using Zucker diabetic Sprague–Dawley (ZDSD) rats was developed to describe this transition through altering insulin sensitivity (IS, %) as a result of accumulating excess body weight and β-cell function (BCF, %) to affect glucose-insulin homeostasis. Body weight, fasting plasma glucose (FPG), and fasting serum insulin (FSI) were collected biweekly over 24 weeks from ZDSD rats (n = 23) starting at age 7 weeks. A semi-mechanistic model previously developed with clinical data was adapted to rat data with BCF and IS estimated relative to humans. Non-linear mixed-effect model estimation was performed using NONMEM. Baseline IS and BCF were 41% compared to healthy humans. BCF was described with a non-linear rise which peaked at 14 weeks before gradually declining to a negligible level. A component for excess growth reflecting obesity was used to affect IS, and a glucose-dependent renal effect exerted a two- to sixfold increase on the elimination of glucose. A glucose-dependent weight loss effect towards the end of experiment was implemented. A semi-mechanistic model to describe the dynamics of glucose and insulin was successfully developed for a rat population, transitioning from healthy to advanced diabetes. It is also shown that weight loss can be modeled to mimic the glucotoxicity phenomenon seen in advanced hyperglycemia.




Similar content being viewed by others
References
Norris SL, Zhang X, Avenell A, Gregg E, Bowman B, Schmid CH, et al. Long-term effectiveness of weight-loss interventions in adults with pre-diabetes: a review. Am J Prev Med. 2005;28(1):126–39.
Karve A, Hayward RA. Prevalence, diagnosis, and treatment of impaired fasting glucose and impaired glucose tolerance in nondiabetic U.S. adults. Diabetes Care. 2010;33(11):2355–9.
Bertram MY, Vos T. Quantifying the duration of pre-diabetes. Aust N Z J Public Health. 2010;34(3):311–4.
Shafrir E. Animal models of non-insulin-dependent diabetes. Diabetes Metab Rev. 1992;8(3):179–208.
Peterson RG, Jackson CV, Zimmerman K, De Winter W, Huebert N, Hansen MK. Characterization of the ZDSD rat: A translational model for the study of metabolic syndrome and type 2 diabetes. J Diabetes Res. 2015;487816.
Reinwald S, Peterson RG, Allen MR, Burr DB. Skeletal changes associated with the onset of type 2 diabetes in the ZDF and ZDSD rodent models. Am J Physiol Endocrinol Metab. 2009;296(4):E765–E74.
Choy S, Kjellsson MC, Karlsson MO, Winter WD. Weight-HbA1c-insulin-glucose model for describing disease progression of type 2 diabetes. CPT: Pharmacometrics Syst Pharmacol. 2015. doi:10.1002/psp4.12051.
Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21(6):697–738.
Macor C, Ruggeri A, Mazzonetto P, Federspil G, Cobelli C, Vettor R. Visceral adipose tissue impairs insulin secretion and insulin sensitivity but not energy expenditure in obesity. Metab Clin Exp. 1997;46(2):123–9.
Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program [3]. Diabetes Care. 1998;21(12):2191–2.
Brody S. Bioenergetics and growth. New York: Reinhold Publishing Corporation; 1945.
Lewis SM, Johnson ZJ, Mayhugh MA, Duffy PH. Nutrient intake and growth characteristics of male Sprague–Dawley rats fed AIN-93M purified diet or NIH-31 natural-ingredient diet in a chronic two-year study. Aging Clin Exp Res. 2003;15(6):460–8.
Elia M, Carter A, Bacon S, Winearls CG, Smith R. Clinical usefulness of urinary 3-methylhistidine excretion in indicating muscle protein breakdown. Br Med J. 1981;282(6261):351–4.
Charlton M, Nair KS. Protein metabolism in insulin-dependent diabetes mellitus. J Nutr. 1998;128(2 SUPPL):323S–7S.
Liao JJZ, Liu R. Re-parameterization of five-parameter logistic function. J Chemom. 2009;23(5):248–53.
de Winter W, DeJongh J, Post T, Ploeger B, Urquhart R, Moules I, et al. A mechanism-based disease progression model for comparison of long-term effects of pioglitazone, metformin and gliclazide on disease processes underlying Type 2 Diabetes Mellitus. J Pharmacokinet Pharmacodyn. 2006;33(3):313–43.
Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–95.
Weir GC, Bonner-Weir S. Five of stages of evolving β-cell dysfunction during progression to diabetes. Diabetes. 2004;53 SUPPL 3:S16–21.
Beal SL, Sheiner LB, Boeckmann AJ, Bauer RJ. NONMEM User’s Guides. Ellicott City, MD, USA: Icon Development Solutions; 1989–2014.
Dosne AG, Bergstrand M, Karlsson MO. Application of sampling importance resampling to estimate parameter uncertainty distributions. Population Approach Group in Europe 2013. p. Abstr 2907 [www.page-meeting.org/?abstract=2907]
Baek SH, Kim EK, Goudreau JL, Lookingland KJ, Kim SW, Yu SW. Insulin withdrawal-induced cell death in adult hippocampal neural stem cells as a model of autophagic cell death. Autophagy. 2009;5(2):277–9.
Rave K, Nosek L, Posner J, Heise T, Roggen K, van Hoogdalem EJ. Renal glucose excretion as a function of blood glucose concentration in subjects with type 2 diabetes - Results of a hyperglycaemic glucose clamp study. Nephrol Dial Transplant. 2006;21(8):2166–71.
Gabriely I, Ma XH, Yang XM, Atzmon G, Rajala MW, Berg AH, et al. Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine-mediated process? Diabetes. 2002;51(10):2951–8.
Kwon H, Pessin JE. Adipokines mediate inflammation and insulin resistance. Frontiers in Endocrinology. [Review]. 2013;4.
Henry RR, Wallace P, Olefsky JM. Effects of weight loss on mechanisms of hyperglycemia in obese non-insulin-dependent diabetes mellitus. Diabetes. 1986;35(9):990–8.
McGuire MT, Wing RR, Klein ML, Hill JO. The behavioral characteristics of individuals who lose weight unintentionally. Obes Res. 1999;7(5):485–90.
Gao W, Bihorel S, Dubois DC, Almon RR, Jusko WJ. Mechanism-based disease progression modeling of type 2 diabetes in Goto-Kakizaki rats. J Pharmacokinet Pharmacodyn. 2011;38(1):143–62.
Campetelli G, Lombarte M, Biset H, Rigalli A, Basualdo MS. A rat-human scale-up procedure for the endocrine system. Comput Chem Eng. 2014;71:512–20.
Eaton DM, Rogers AN, Rice AE, Coy K, Peterson RG, Packer CS. Relationship between dyslipidemia and hypertension (HBP) in male ZDSD, a new rat model of metabolic syndrome (MetS). FASEB J. 2013;27:1190.1.
Acknowledgments
Steve Choy’s doctoral studies are supported by Janssen Pharmaceutica.
The authors would like to thank Richard Peterson (PreClinOmics, Inc) for supplying the experimental data used in the analysis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(TXT 10 kb)
Appendix I
Appendix I
(a) Derivation of the Brody Growth Function as Differential Equation
The Brody growth function is formulated as
where WGT max is the animal’s maximum weight, WGT 0 is the animal’s birth weight, and k is the growth rate of the animal per unit of time t.
The Brody growth function is transformed to a differential equation for use in model analysis through the following proof:
Let
Subtract a from both sides of (1):
Multiply both sides of (3) by -1:
Substitute (4) into (2) to obtain:
(b) Derivation of the Natural Growth Rate of Brody Function
The Brody growth function is formulated as
where WGT max is the animal’s maximum weight, WGT 0 is the animal’s birth weight, and k is the growth rate of the animal per unit of time t.
We are interested in calculating the natural growth rate k, which is achieved by rearranging the Brody growth function as follows:
The rats were at age 7 weeks at the start of experiment. Values of WGTmax and WGT0 are obtained from the literature (12), and weight at 7 weeks is equal to the observed baseline weight (BLWT).
Substituting WGT max = 840g, WGT(7) = BLWT = 220g, WGT 0 = 6.1g, t = 7, we get:
(c) Derivation of the Steady-State Quadratic Solution from Differential Equations of FSI and FPG
Assuming steady-state,
Rearranging Eq.2 for FPG0 (Note: KinFPG / KoutFPG = KIOG and EFUrine, EFS, EFB = 1 at baseline)
Substitute FPG from Eq.3 back to Eq. 1
Expand the brackets (Note: KinFSI/KoutFSI = KIOI)
Rearranging to get the quadratic equation form ax 2 + bx + c = 0
FSI0 can now be solved using the quadratic formula:
Note: only the positive root is of interest.
Substitute FSI0 back into Eq 3 to calculate FPG0:
Rights and permissions
About this article
Cite this article
Choy, S., de Winter, W., Karlsson, M.O. et al. Modeling the Disease Progression from Healthy to Overt Diabetes in ZDSD Rats. AAPS J 18, 1203–1212 (2016). https://doi.org/10.1208/s12248-016-9931-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-016-9931-0