Abstract
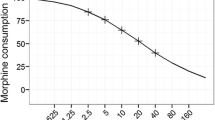
Joint analysis of pain intensity and opioid consumption is encouraged in trials of postoperative pain. However, previous approaches have not appropriately addressed the complexity of their interrelation in time. In this study, we applied a non-linear mixed effects model to simultaneously study pain intensity and opioid consumption in a 4-h postoperative period for 44 patients undergoing percutaneous kidney stone surgery. Analysis was based on 748 Numerical Rating Scale (NRS) scores of pain intensity and 51 observed morphine and oxycodone dosing events. A joint model was developed to describe the recurrent pattern of four key phases determining the development of pain intensity and opioid consumption in time; (A) Distribution of pain intensity scores which followed a truncated Poisson distribution with time-dependent mean score ranging from 0.93 to 2.45; (B) Probability of transition to threshold pain levels (NRS ≥ 3) which was strongly dependent on previous pain levels ranging from 2.8–15.2% after NRS of 0–2; (C) Probability of requesting opioid when allowed (NRS ≥ 3) which was strongly correlated with the number of previous doses, ranging from 89.8% for requesting the first dose to 26.1% after three previous doses; (D) Reduction in pain scores after opioid dosing which was significantly related to the pain intensity at time of opioid request (P < 0.001). This study highlights the importance of analyzing pain intensity and opioid consumption in an integrated manner. Non-linear mixed effects modeling proved a valuable tool for analysis of interventions that affect pain intensity, probability of rescue dosing or the effect of opioids in the postoperative pain period.








Similar content being viewed by others
References
McQuay HJ, Derry S, Eccleston C, Wiffen PJ, Andrew MR. Evidence for analgesic effect in acute pain—50 years on. Pain. 2012;153(7):1364–7. doi:10.1016/j.pain.2012.01.024.
Woolf CJ. Overcoming obstacles to developing new analgesics. Nat Med. 2010;16(11):1241–7. doi:10.1038/nm.2230.
Cousins MJ, Brennan F, Carr DB. Pain relief: a universal human right. Pain. 2004;112(1-2):1–4. doi:10.1016/j.pain.2004.09.002.
Gilron I, Jensen MP. Clinical trial methodology of pain treatment studies: selection and measurement of self-report primary outcomes for efficacy. Reg Anesth Pain Med. 2011;36(4):374–81. doi:10.1097/AAP.0b013e318217a635.
US Food Drug Administration. Guidance for industry analgesic indications: developing drug and biological products. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM384691.pdf [accessed 2015 Mar 12]. 2014.
Sechzer PH. Objective measurement of pain. Anesthesiology. 1968;29(1):209–10.
McQuay HJ, Poon KH, Derry S, Moore RA. Acute pain: combination treatments and how we measure their efficacy. Br J Anaesth. 2008;101(1):69–76. doi:10.1093/bja/aen108.
Kissin I. Patient-controlled-analgesia analgesimetry and its problems. Anesth Analg. 2009;108(6):1945–9. doi:10.1213/ane.0b013e3181a1a481.
Dahl JB, Nielsen RV, Wetterslev J, Nikolajsen L, Hamunen K, Kontinen VK, et al. Post-operative analgesic effects of paracetamol, NSAIDs, glucocorticoids, gabapentinoids and their combinations: a topical review. Acta Anaesthesiol Scand. 2014;58(10):1165–81. doi:10.1111/aas.12382.
Ong CK, Lirk P, Seymour RA, Jenkins BJ. The efficacy of preemptive analgesia for acute postoperative pain management: a meta-analysis. Anesth Analg. 2005;100(3):757–73. doi:10.1213/01.ANE.0000144428.98767.0E.
Brennan TJ. Pathophysiology of postoperative pain. Pain. 2011;152(3 Suppl):S33–40. doi:10.1016/j.pain.2010.11.005.
Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain. 2011;152(10):2399–404. doi:10.1016/j.pain.2011.07.005.
Jensen MP, Tome-Pires C, Sole E, Racine M, Castarlenas E, de la Vega R, et al. Assessment of pain intensity in clinical trials: individual ratings vs composite scores. Pain Med. 2015;16(1):141–8. doi:10.1111/pme.12588.
Plan EL, Elshoff JP, Stockis A, Sargentini-Maier ML, Karlsson MO. Likert pain score modeling: a Markov integer model and an autoregressive continuous model. Clin Pharmacol Ther. 2012;91(5):820–8. doi:10.1038/clpt.2011.301.
Macintyre PE. Safety and efficacy of patient-controlled analgesia. Br J Anaesth. 2001;87(1):36–46.
Juul RV, Rasmussen S, Kreilgaard M, Christrup LL, Simonsson USH, Lund TM. Repeated time-to-event analysis of consecutive analgesic events in postoperative pain. Anesthesiology. 2015;123(12):1411–9.
Hu YJ, Ku TH, Jan RH, Wang K, Tseng YC, Yang SF. Decision tree-based learning to predict patient controlled analgesia consumption and readjustment. BMC Med Inform Decis Mak. 2012;12:131. doi:10.1186/1472-6947-12-131.
Sheiner LB, Rosenberg B, Marathe VV. Estimation of population characteristics of pharmacokinetic parameters from routine clinical data. J Pharmacokinet Biopharm. 1977;5(5):445–79.
Upton RN, Mould DR. Basic concepts in population modeling, simulation, and model-based drug development: part 3-introduction to pharmacodynamic modeling methods. CPT: Pharm Syst Pharmacol. 2014;3:e88. doi:10.1038/psp.2013.71.
Beal S, Sheiner L, Boeckmann A, Bauer R. NONMEM 7.3. 0 users guide. Hanover: Icon Development Solutions; 2014.
Björnsson MA, Simonsson USH. Modelling of pain intensity and informative dropout in a dental pain model after naproxcinod, naproxen and placebo administration. Br J Clin Pharmacol. 2011;71(6):899–906. doi:10.1111/j.1365-2125.2011.03924.x.
Sheiner LB. A new approach to the analysis of analgesic drug trials, illustrated with bromfenac data. Clin Pharmacol Ther. 1994;56(3):309–22.
Juul RV, Nyberg J, Lund TM, Rasmussen S, Kreilgaard M, Christrup LL, et al. A pharmacokinetic-pharmacodynamic model of morphine exposure and subsequent morphine consumption in postoperative pain. Pharm Res. 2016. doi:10.1007/s11095-015-1853-5.
Pedersen KV, Olesen AE, Drewes AM, Osther PJ. Morphine versus oxycodone analgesia after percutaneous kidney stone surgery: a randomised double blinded study. Urolithiasis. 2013;41(5):423–30. doi:10.1007/s00240-013-0587-2.
Silvasti M, Rosenberg P, Seppala T, Svartling N, Pitkanen M. Comparison of analgesic efficacy of oxycodone and morphine in postoperative intravenous patient-controlled analgesia. Acta Anaesthesiol Scand. 1998;42(5):576–80.
R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2014.
Wickham H. ggplot2: elegant graphics for data analysis. New York: Springer; 2009.
Wickham H, Francois. R. dplyr: a grammar of data manipulation. R package version 0.2. Available at http://CRAN.R-project.org/package=dplyr. 2014.
Plan EL. Modeling and simulation of count data. CPT: Pharmacometrics Syst Pharmacol. 2014;3:e129. doi:10.1038/psp.2014.27.
Silverman DG, O'Connor TZ, Brull SJ. Integrated assessment of pain scores and rescue morphine use during studies of analgesic efficacy. Anesth Analg. 1993;77(1):168–70.
Sverrisdottir E, Lund TM, Olesen AE, Drewes AM, Christrup LL, Kreilgaard M. A review of morphine and morphine-6-glucuronide's pharmacokinetic-pharmacodynamic relationships in experimental and clinical pain. Eur J Pharm Sci : Off J Eur Fed Pharm Sci. 2015;74:45–62. doi:10.1016/j.ejps.2015.03.020.
Staahl C, Upton R, Foster DJ, Christrup LL, Kristensen K, Hansen SH, et al. Pharmacokinetic-pharmacodynamic modeling of morphine and oxycodone concentrations and analgesic effect in a multimodal experimental pain model. J Clin Pharmacol. 2008;48(5):619–31. doi:10.1177/0091270008314465.
Juul RV, Foster DJ, Upton RN, Andresen T, Graversen C, Drewes AM, et al. Pharmacodynamic modelling of placebo and buprenorphine effects on event-related potentials in experimental pain. Basic Clin Pharmacol Toxicol. 2014;115(4):343–51. doi:10.1111/bcpt.12217.
Acknowledgments
The study was supported by an unrestricted grant from Norpharma A/S. Mech-Sense, Aalborg University Hospital has received funding from Innovation Fund Denmark for Strategic Research in Individuals, Disease and Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
Asbjørn Mohr Drewes has received unrestricted research grants from Mundipharma, AstraZeneca, Lundbeck, and Pfizer and served as a Consultant/Advisory Board member for Mundipharma, AstraZeneca, Almirall, and Shire. Palle Jørn Sloth Osther has received an unrestricted research grant from Norpharma A/S. Rasmus Vestergaard Juul, Katrine Rørbæk Knøsgaard, Anne Estrup Olesen, Katja Venborg Pedersen, Mads Kreilgaard, Lona Louring Christrup, and Trine Meldgaard Lund have no conflict of interest to declare.
Additional information
Rasmus V Juul and Katrine R Knøsgaard contributed equally to this work.
Rights and permissions
About this article
Cite this article
Juul, R.V., Knøsgaard, K.R., Olesen, A.E. et al. A Model-Based Approach for Joint Analysis of Pain Intensity and Opioid Consumption in Postoperative Pain. AAPS J 18, 1013–1022 (2016). https://doi.org/10.1208/s12248-016-9921-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-016-9921-2