Abstract
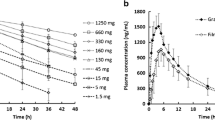
Intraoral (IO) delivery is an alternative administration route to deliver a drug substance via the mouth that provides several advantages over conventional oral dosage forms. The purpose of this work was to develop and evaluate a novel, physiologically based oral cavity model for projection and mechanistic analysis of the clinical pharmacokinetics of intraoral formulations. The GastroPlus™ Oral Cavity Compartmental Absorption and Transit (OCCAT™) model was used to simulate the plasma concentration versus time profiles and the fraction and rate of intraoral drug transit/absorption for Intermezzo® sublingual tablets (zolpidem tartrate). The model was evaluated by the goodness-of-fit between simulated and observed concentrations and the deviation of key PK parameters (e.g., C max, T max, and AUC). In addition, a sensitivity analysis was conducted to demonstrate the interplay and impact of key modeling parameters on the fraction absorbed via oral mucosa (F a_IO). The OCCAT™ model captured the observed pharmacokinetics for Intermezzo® sublingual tablets (R 2 > 0.9). The predicted deviations (%) for C max, AUC0–inf, AUC0–20 min, and T max were 5.7, 28.0, 11.8, and 28.6%, respectively, indicating good prediction accuracy. The model also estimated ~18% of total drug was absorbed via the IO route. Furthermore, the sensitivity analysis indicated that the F a_IO was not only associated with drug diffusivity and unbound fraction in epithelium tissue (f ut) but also depended on the physicochemical properties of compounds for IO delivery (e.g., solubility and logD pH = 7.4). The novel physiologically based IO absorption OCCAT™ model showed satisfactory performance and will be helpful to guide development of future intraoral formulations.





Similar content being viewed by others
References
Mathias NR, Hussain MA. Non-invasive systemic drug delivery: developability considerations for alternate routes of administration. J Pharm Sci. 2010;99(1):1–20.
Patel VF, Liu F, Brown MB. Advances in oral transmucosal drug delivery. J Control Release. 2011;153(2):106–16.
Hearnden V, Sankar V, Hull K, Juras DV, Greenberg M, Kerr AR, et al. New developments and opportunities in oral mucosal drug delivery for local and systemic disease. Adv Drug Deliv Rev. 2012;64(1):16–28.
Sugano K. Introduction to computational oral absorption simulation. Expert Opin Drug Metab Toxicol. 2009;5(3):259–93.
Kostewicz ES, Aarons L, Bergstrand M, Bolger MB, Galetin A, Hatley O, et al. PBPK models for the prediction of in vivo performance of oral dosage forms. Eur J Pharm Sci. 2013 Sep 21.
Kates RE. Absorption kinetics of sublingually administered propranolol. J Med. 1977;8(6):393–402.
Beckett ABR, Triggs EJ. Kinetics of buccal absorption of amphetamines. J Pharm Pharmacol. 1968;20(2):92–7.
Wang Y, Wang Z, Zuo Z, Tomlinson B, Lee BT, Bolger MB, et al. Clinical pharmacokinetics of buffered propranolol sublingual tablet (Promptol)—application of a new “physiologically based” model to assess absorption and disposition. AAPS J. 2013;15(3):787–96.
Shankaran H, Adeshina F, Teeguarden JG. Physiologically-based pharmacokinetic model for Fentanyl in support of the development of provisional advisory levels. Toxicol Appl Pharmacol. 2013;273(3):464–76.
Bartlett JA, van der Voort MK. Understanding the oral mucosal absorption and resulting clinical pharmacokinetics of asenapine. AAPS PharmSciTech. 2012;13(4):1110–5.
Zhang H, Zhang J, Streisand JB. Oral mucosal drug delivery: clinical pharmacokinetics and therapeutic applications. Clin Pharmacokinet. 2002;41(9):661–80.
Squier CA, Wertz PW. Permeability and the pathophysiology of oral mucosa. Adv Drug Deliv Rev. 1993;12:13–24.
Goswami T, Kokate A, Jasti BR, Li X. In silico model of drug permeability across sublingual mucosa. Arch Oral Biol. 2012;58(5):545–51.
Agoram B, Woltosz WS, Bolger MB. Predicting the impact of physiological and biochemical processes on oral drug bioavailability. Adv Drug Deliv Rev. 2001;50 Suppl 1:S41–67.
Lagerlof F, Dawes C. The volume of saliva in the mouth before and after swallowing. J Dent Res. 1984;63(5):618–21.
Ramirez E, Laosa O, Guerra P, Duque B, Mosquera B, Borobia AM, et al. Acceptability and characteristics of 124 human bioequivalence studies with active substances classified according to the biopharmaceutic classification system. Br J Clin Pharmacol. 2010;70(5):694–702.
Durand A, Thenot JP, Bianchetti G, Morselli PL. Comparative pharmacokinetic profile of two imidazopyridine drugs: zolpidem and alpidem. Drug Metab Rev. 1992;24(2):239–66.
FDA. Intermezzo NDA files. 2011.
Staner L, Eriksson M, Cornette F, Santoro F, Muscat N, Luthinger R, et al. Sublingual zolpidem is more effective than oral zolpidem in initiating early onset of sleep in the post-nap model of transient insomnia: a polysomnographic study. Sleep Med. 2009;10(6):616–20.
Staner C, Joly F, Jacquot N, Vlasova ID, Nehlin M, Lundqvist T, et al. Sublingual zolpidem in early onset of sleep compared to oral zolpidem: polysomnographic study in patients with primary insomnia. Curr Med Res Opin. 2010;26(6):1423–31.
Ho NFH. Biophysical kinetic modeling of buccal absorption. Adv Drug Deliv Rev. 1993;12(1–2):61–97.
Rathbone MJ, Hadgraft J. Absorption of drugs from the human oral cavity. Int J Pharm. 1991;74:9–24.
Lien EJCK, Tong RT, George L. Physicochemical properties, bioavailability of drugs: buccal and percutaneous absorptions. Drug Intelligence. 1971;5(2):38–41.
Beckett AH, Moffat AC. Correlation of partition coefficients in n-heptane-aqueous systems with buccal absorption data for a series of amines and acids. J Pharm Pharmacol. 1969;21(Suppl):144S.
Greenblatt DJ, Harmatz JS, Roth T, Singh NN, Moline ML, Harris SC, et al. Comparison of pharmacokinetic profiles of zolpidem buffered sublingual tablet and zolpidem oral immediate-release tablet: results from a single-center, single-dose, randomized, open-label crossover study in healthy adults. Clin Ther. 2013;35(5):604–11.
Green R, Hicks RW. Orally disintegrating vardenafil tablets for the treatment of erectile dysfunction: efficacy, safety, and patient acceptability. Patient Prefer Adherence. 2011;5:181–5.
Heckmann SM, Heckmann JG, Hilz MJ, Popp M, Marthol H, Neundorfer B, et al. Oral mucosal blood flow in patients with burning mouth syndrome. Pain. 2001;90:281–6.
Atasever NE, Ercan MT, Naldoken S, Ulutuncel N. Effect of wearing complete dentures on human palatal mucosal blood flow measured by 133-Xe clearance. Arch Oral Biol. 1991;36(9):627–30.
Collins LMC, Dawes C. The surface area of the adult human mouth and thickness of the salivary film covering the teeth and oral mucosa. J Dent Res. 1987;66(8):1300–2.
Sudhakar Y, Kuotsu K, Bandyopadhayay AK. Buccal bioadhesive drug delivery—a promising option for orally less efficient drugs. J Control Release. 2006;114(1):15–40.
Vorperian HK, Wang S, Chung MK, Schimek EM, Durtschi RB, Kent RD, et al. Anatomic development of the oral and pharyngeal portions of the vocal tract: an imaging study. J Acoust Soc Am. 2009;125(3):1666–78.
Feldchtein FI, Gelikonov GV, Gelikonov VM, Iksanov RR, Kuranov RV, Sergeev AM, et al. In vivo OCT imaging of hard and soft tissue of the oral cavity. Opt Express. 1998;3(6):239–50.
Prestin S, Rothschild SI, Betz CS, Kraft M. Measurement of epithelial thickness within the oral cavity using optical coherence tomography. Head Neck. 2012;34(12):1777–81.
Klein-Szanto AJP, Schroeder HE. Architecture and density of the connective tissue papillae of the human oral mucosa. J Anat. 1977;123(1):93–109.
Muller HP, Schaller N, Eger T, Heinecke A. Thickness of masticatory mucosa. J Clin Periodontol. 2000;27:431–6.
Valentine JA, Scott J, West CR, Hill CAS. A histological analysis of the early effects of alcohol and tobacco usage on human lingual epithelium. J Oral Pathol. 1985;14:654–65.
Sasaki M. Histomorphometric analysis of age-related changes in epithelial thickness and Langerhans cell density of the human tongue. Tohoku J Exp Med. 1994;173:321–36.
Aframian DJ, Davidowitz T, Benoliel R. The distribution of oral mucosal pH values in healthy saliva secretors. Oral Dis. 2006;12:420–3.
FDA. Asenapine FDA NDA file. 2009.
Berk SI, Beckman K, Hoon TJ, Hariman RJ, Hu D, Siegel FP, et al. Comparison of the pharmacokinetics and electrocardiographic effects of sublingual and intravenous verapamil. Pharmacotherapy. 1992;12(1):33–9.
Hukkanen J, Jacob 3rd P, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57(1):79–115.
Molander L, Lunell E. Pharmacokinetic investigation of a nicotine sublingual tablet. Eur J Clin Pharmacol. 2001;56(11):813–9.
Acknowledgments
The authors would like to thank Anita Lalloo, Becky Nissley, Kimberly Manser, and Poonam Saraf for their assistance in the in vitro permeation studies; Henry Wu and David Edward Storey for scientific input and discussion; and Merck creative studios for English edits.
Author information
Authors and Affiliations
Corresponding author
Additional information
Binfeng Xia and Zhen Yang equally contributed to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 94.7 kb)
Rights and permissions
About this article
Cite this article
Xia, B., Yang, Z., Zhou, H. et al. Development of a Novel Oral Cavity Compartmental Absorption and Transit Model for Sublingual Administration: Illustration with Zolpidem. AAPS J 17, 631–642 (2015). https://doi.org/10.1208/s12248-015-9727-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-015-9727-7