Abstract
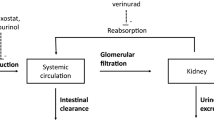
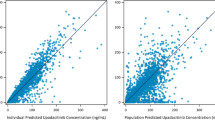
ABT-639 is a selective T-type calcium channel blocker with efficacy in a wide range of preclinical models of nociceptive and neuropathic pain. In the current first-in-human (FIH) study, the pharmacokinetics, tolerability, and safety of ABT-639 after single- (up to 170 mg) and multiple doses (up to 160 mg BID) were evaluated in healthy volunteers in a randomized, double-blinded, placebo-controlled manner. ABT-639 demonstrated acceptable safety and pharmacokinetic profiles in human. Results from assessment of the routine laboratory variables showed an unexpected statistically significant and clinically relevant decrease in blood uric acid with the increase in ABT-639 dose, which is possibly due to inhibition in URAT1 transporter. Pharmacokinetic/pharmacodynamic models were constructed to characterize the relationship between ABT-639 exposure and uric acid response. The final model was a mechanism-based indirect response pharmacodynamic model with the stimulation of uric acid elimination by ABT-639. The model estimated K in values in males and females were 10.2 and 7.13 μmol/h, respectively. The model estimated K out was 0.033 1/h. ABT-639 concentration that can produce 50% stimulation in uric acid elimination was estimated to be 8,070 ng/mL. Based on the final model, further simulations were conducted to predict the effect of ABT-639 on uric acid in gout patients. The simulation results indicated that, if the urate-lowering response to ABT-639 in gout patients is similar to that in healthy subjects, ABT-639 BID doses of 140 mg or higher would be expected to provide clinically meaningful lowering of blood uric acid levels below the 380 μmol/L solubility limit of monosodium urate.





Similar content being viewed by others
References
Jarvis MF, Scott VE, McGaraughty S, Chu KL, Xu J, Niforatos W, et al. A peripherally acting, selective T-type calcium channel blocker, ABT-639, effectively reduces nociceptive and neuropathic pain in rats. Biochemical pharmacology. 2014;89(4):536–44. doi:10.1016/j.bcp.2014.03.015.
Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH, et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 2002;417(6887):447–52. doi:10.1038/nature742.
Anzai N, Kanai Y, Endou H. New insights into renal transport of urate. Curr Opin Rheumatol. 2007;19(2):151–7. doi:10.1097/BOR.0b013e328032781a.
Perez-Ruiz F, Alonso-Ruiz A, Calabozo M, Herrero-Beites A, Garcia-Erauskin G, Ruiz-Lucea E. Efficacy of allopurinol and benzbromarone for the control of hyperuricaemia. A pathogenic approach to the treatment of primary chronic gout. Ann Rheum Dis. 1998;57(9):545–9.
Kelley WN. Current therapy of gout and hyperuricemia. Hosp Pract. 1976;11(5):69–76.
Benedict JD, Forsham PH, Stetten Jr D. The metabolism of uric acid in the normal and gouty human studied with the aid of isotopic uric acid. J Biol Chem. 1949;181(1):183–93.
Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 63(10):3136–41. doi: 10.1002/art.30520.
Terkeltaub R, Bushinsky DA, Becker MA. Recent developments in our understanding of the renal basis of hyperuricemia and the development of novel antihyperuricemic therapeutics. Arthritis Res Ther. 2006;8 Suppl 1:S4. doi:10.1186/ar1909.
Richette P, Bardin T. Gout. Lancet. 2010;375(9711):318–28. doi:10.1016/S0140-6736(09)60883-7.
Zhang W, Doherty M, Bardin T, Pascual E, Barskova V, Conaghan P, et al. EULAR evidence based recommendations for gout. Part II: management. Report of a task force of the EULAR standing committee for international clinical studies including therapeutics (ESCISIT). Ann Rheum Dis. 2006;65(10):1312–24. doi:10.1136/ard.2006.055269.
Becker MA, Schumacher Jr HR, Wortmann RL, MacDonald PA, Eustace D, Palo WA. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353(23):2450–61. doi:10.1056/NEJMoa050373.
Hair PI, McCormack PL, Keating GM. Febuxostat. Drugs. 2008;68(13):1865–74.
Ogryzlo MA, Harrison J. Evaluation of uricosuric agents in chronic gout. Ann Rheum Dis. 1957;16(4):425–37.
Hande KR, Noone RM, Stone WJ. Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med. 1984;76(1):47–56.
Fam AG. Difficult gout and new approaches for control of hyperuricemia in the allopurinol-allergic patient. Curr Rheumatol Rep. 2001;3(1):29–35.
Emmerson BT. The management of gout. N Engl J Med. 1996;334(7):445–51. doi:10.1056/NEJM199602153340707.
Anzai N, Enomoto A, Endou H. Renal urate handling: clinical relevance of recent advances. Curr Rheumatol Rep. 2005;7(3):227–34.
Acknowledgments
AbbVie contributed to the study design, research, and interpretation of data, writing, reviewing, and approving the publication. Wei Liu, Rachel Duan, Walid Awni, and Sandeep Dutta are AbbVie employees and may hold AbbVie stocks or options. Guohua An and Wolfram Nothaft are former AbbVie employees and have no further conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
An, G., Liu, W., Duan, W.R. et al. Population Pharmacokinetics and Exposure-Uric Acid Analyses After Single and Multiple Doses of ABT-639, a Calcium Channel Blocker, in Healthy Volunteers. AAPS J 17, 416–426 (2015). https://doi.org/10.1208/s12248-014-9709-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-014-9709-1