Abstract
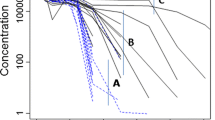
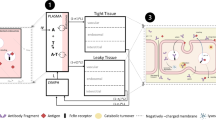
A mathematical pharmacokinetic/anti-drug-antibody (PK/ADA) model was constructed for quantitatively assessing immunogenicity for therapeutic proteins. The model is inspired by traditional pharmacokinetic/pharmacodynamic (PK/PD) models, and is based on the observed impact of ADA on protein drug clearance. The hypothesis for this work is that altered drug PK contains information about the extent and timing of ADA generation. By fitting drug PK profiles while accounting for ADA-mediated drug clearance, the model provides an approach to characterize ADA generation during the study, including the maximum ADA response, sensitivity of ADA response to drug dose level, affinity maturation rate, time lag to observe an ADA response, and the elimination rate for ADA–drug complex. The model also provides a mean to estimate putative concentration–time profiles for ADA, ADA–drug complex, and ADA binding affinity-time profile. When simulating ADA responses to various drug dose levels, bell-shaped dose–response curves were generated. The model contains simultaneous quantitative modeling and provides estimation of the characteristics of therapeutic protein drug PK and ADA responses in vivo. With further experimental validation, the model may be applied to the simulation of ADA response to therapeutic protein drugs in silico, or be applied in subsequent PK/PD models.









Similar content being viewed by others
REFERENCES
Chirmule N, Jawa V, Meibohm B. Immunogenicity to therapeutic proteins: impact on PK/PD and Efficacy. AAPS Journal. 2012;14(2):296–302. doi:10.1208/s12248-012-9340-y.
Barbosa MD. Immunogenicity of biotherapeutics in the context of developing biosimilars and biobetters. Drug Discov Today. 2011;16(7–8):345–53.
Schellekens H. Factors influencing the immunogenicity of therapeutic proteins. Nephrol Dial Transplant. 2005;20 Suppl 6:vi3–9.
Shankar G, Shores E, Wagner C, Mire-Sluis A. Scientific and regulatory considerations on the immunogenicity of biologics. Trends Biotechnol. 2006;24(6):274–80.
Pendley C, Schantz A, Wagner C. Immunogenicity of therapeutic monoclonal antibodies. Curr Opin Mol Ther. 2003;5(2):172–9.
US Department of Health and Human Services Food and Drug Administration. Guidance for industry assay development for immunogenicity testing of therapeutic proteins. 2009.
US Department of Health and Human Services Food and Drug Administration. Guidance for industry premarketing risk assessment. 2004.
US Department of Health and Human Services Food and Drug Administration. Guidance for industry immunotoxicology evaluation of investigational new drugs. 2002.
Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84(5):548–58.
Ponce R, Abad L, Amaravadi L, Gelzleichter T, Gore E, Green J, et al. Immunogenicity of biologically-derived therapeutics: assessment and interpretation of nonclinical safety studies. Regul Toxicol Pharmacol. 2009;54(2):164–82.
Casadevall N, Nataf J, Viron B, Kolta A, Kiladjian JJ, Martin-Dupont P, et al. Pure red-cell aplasia and antierythropoietin antibodies in patients treated with recombinant erythropoietin. N Engl J Med. 2002;346(7):469–75.
Li J, Yang C, Xia Y, Bertino A, Glaspy J, Roberts M, et al. Thrombocytopenia caused by the development of antibodies to thrombopoietin. Blood. 2001;98(12):3241–8.
Gupta S, Indelicato SR, Jethwa V, Kawabata T, Kelley M, Mire-Sluis AR, et al. Recommendations for the design, optimization, and qualification of cell-based assays used for the detection of neutralizing antibody responses elicited to biological therapeutics. J Immunol Methods. 2007;321(1–2):1–18.
Mire-Sluis AR, Barrett YC, Devanarayan V, Koren E, Liu H, Maia M, et al. Recommendations for the design and optimization of immunoassays used in the detection of host antibodies against biotechnology products. J Immunol Methods. 2004;289(1–2):1–16.
Shankar G, Pendley C, Stein KE. A risk-based bioanalytical strategy for the assessment of antibody immune responses against biological drugs. Nat Biotechnol. 2007;25(5):555–61.
Neuberger MS, Ehrenstein MR, Rada C, Sale J, Batista FD, Williams G, et al. Memory in the B-cell compartment: antibody affinity maturation. Philos Trans R Soc Lond B Biol Sci. 2000;355(1395):357–60.
Bell GI. Mathematical model of clonal selection and antibody production. J Theor Biol. 1970;29(2):191–232.
Lee HY, Topham DJ, Park SY, Hollenbaugh J, Treanor J, Mosmann TR, et al. Simulation and prediction of the adaptive immune response to influenza A virus infection. J Virol. 2009;83(14):7151–65.
Xu ZH, Lee H, Vu T, Hu C, Yan H, Baker D, et al. Population pharmacokinetics of golimumab in patients with ankylosing spondylitis: impact of body weight and immunogenicity. Int J Clin Pharmacol Ther. 2010;48(9):596–607.
Bonate PL, Sung C, Welch K, Richards S. Conditional modeling of antibody titers using a zero-inflated poisson random effects model: application to Fabrazyme. J Pharmacokinet Pharmacodyn. 2009;36(5):443–59. Epub 2009/10/01.
Perez Ruixo JJ, Ma P, Chow AT. The utility of modeling and simulation approaches to evaluate immunogenicity effect on the therapeutic protein pharmacokinetics. AAPS Journal. 2012;15(1):172–82. doi:10.1208/s12248-012-9424-8.
Mager DE, Jusko WJ. General pharmacokinetic model for drugs exhibiting target-mediated drug disposition. J Pharmacokinet Pharmacodyn. 2001;28(6):507–32.
Wang YM, Krzyzanski W, Doshi S, Xiao JJ, Perez-Ruixo JJ, Chow AT. Pharmacodynamics-mediated drug disposition (PDMDD) and precursor pool lifespan model for single dose of romiplostim in healthy subjects. AAPS J. 2010;12(4):729–40. Epub 2010/10/22.
Krzyzanski W. Interpretation of transit compartments pharmacodynamic models as lifespan based indirect response models. J Pharmacokinet Pharmacodyn. 2011;38(2):179–204.
Tarlinton DM, Smith KG. Dissecting affinity maturation: a model explaining selection of antibody-forming cells and memory B cells in the germinal centre. Immunol Today. 2000;21(9):436–41.
Fan H, Conner RF, Villarreal LP. Aids: science and society. 6th ed. Boston: Jones and Bartlett; 2011. 30 pages.
Chiras DD. Human biology. 7th ed. Boston: Jones and Bartlett; 2011.
Foote J, Milstein C. Kinetic maturation of an immune response. Nature. 1991;352(6335):530–2.
Davie JM, Paul WE. Receptors on immunocompetent cells. V. Cellular correlates of the “maturation” of the immune response. J Exp Med. 1972;135(3):660–74.
Steiner LA, Eisen HN. Sequential changes in the relative affinity of antibodies synthesized during the immune response. J Exp Med. 1967;126(6):1161–83.
Challacombe SJ, Russell MW. Estimation of the intravascular half-lives of normal rhesus monkey IgG, IgA, and IgM. Immunology. 1979;36(2):331–8.
Foote J, Eisen HN. Kinetic and affinity limits on antibodies produced during immune responses. Proc Natl Acad Sci U S A. 1995;92(5):1254–6.
Batista FD, Neuberger MS. Affinity dependence of the B cell response to antigen: a threshold, a ceiling, and the importance of off-rate. Immunity. 1998;8(6):751–9.
Eisen HN, Siskind GW. Variations in affinities of antibodies during the immune response. Biochemistry. 1964;3:996–1008.
Reth M, Hammerling GJ, Rajewsky K. Analysis of the repertoire of anti-NP antibodies in C57BL/6 mice by cell fusion. I. Characterization of antibody families in the primary and hyperimmune response. Eur J Immunol. 1978;8(6):393–400.
Gearhart PJ, Johnson ND, Douglas R, Hood L. IgG antibodies to phosphorylcholine exhibit more diversity than their IgM counterparts. Nature. 1981;291(5810):29–34.
Flores MV, Hickling TP, Sreckovic S, Fidock MD, Horscroft N, Katragadda M, et al. Preclinical studies of PF-04849285, an interferon-alpha8 fusion protein for the treatment of HCV. Antivir Ther. 2012;17(5):869–81.
Zager MG, Barton HA. A multiscale, mechanism-driven, dynamic model for the effects of 5 alpha-reductase inhibition on prostate maintenance. PLoS One. 2012;7(9):e44359.
Lee E, Salic A, Kruger R, Heinrich R, Kirschner MW. The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS biology. 2003;1(1):E10.
Pier G, Lyczak JB. Immunology, infection, and immunity. Washington: ASM Press; 2004. p. 196–7.
Pathak S, Palan U. Immunology: essential and fundamental. New Delhi: Capital Publishing Company; 2005. p. 82.
Basir S. Textbook of immunology. New Delhi: Prentice Hall of India; 2010. p. 45.
Flower DR. Immunoinformatics and the in silico prediction of immunogenicity. An introduction. Methods Mol Biol. 2007;409:1–15.
Bryson CJ, Jones TD, Baker MP. Prediction of immunogenicity of therapeutic proteins: validity of computational tools. BioDrugs. 2010;24(1):1–8.
Devey ME, Bleasdale-Barr KM, Bird P, Amlot PL. Antibodies of different human IgG subclasses show distinct patterns of affinity maturation after immunization with keyhole limpet haemocyanin. Immunology. 1990;70(2):168–74.
Poulsen TR, Jensen A, Haurum JS, Andersen PS. Limits for antibody affinity maturation and repertoire diversification in hypervaccinated humans. J Immunol. 2011;187(8):4229–35.
Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol. 2001;2(9):816–22. Epub 2001/08/30.
Gorovits B. Antidrug antibody assay validation: industry survey results. AAPS J. 2009;11(1):133–8.
ACKNOWLEDGMENTS
We would like to thank the anonymous reviewers, Scott Fountain, Kenneth Luu, Mary Spilker, and Michael Zager for their valuable suggestions. We thank the PDM department colleagues and especially the immunogenicity strategy group (Li Xue, Bonita Rup, Pratap Singh, John Harrold, Denise O'Hara, Boris Gorovits, Jim McNally, Mengmeng Wang, Anup Zutshi, Ryan Nolan, Michel Awwad, and Anson Abraham) for their help and suggestions.
Conflict of Interest
All authors are employed by Pfizer Inc. This work was partially presented as a poster presentation at the 2012 AAPS National Biotechnology Conference, May 21–22 in San Diego, CA, USA. P. V. receives royalties from the University of Washington Center for Commercialization, the licensor of the SAAM II software.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, X., Hickling, T., Kraynov, E. et al. A Mathematical Model of the Effect of Immunogenicity on Therapeutic Protein Pharmacokinetics. AAPS J 15, 1141–1154 (2013). https://doi.org/10.1208/s12248-013-9517-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-013-9517-z