Abstract
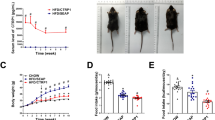
Macrophage infiltration in adipose tissue is strongly correlated with obesity. The exact role of macrophage in the development of obesity, however, has not been fully understood. In this study, using intraperitoneal injection of clodronate liposomes, we tissue-specifically depleted visceral adipose tissue macrophages (VATMs) and explored their roles in initiation and progression of obesity. Two sets of experiments were conducted, using mice on a high-fat diet as the animal model. Mice were injected with clodronate liposomes at the beginning of high-fat diet feeding to investigate the role of VATMs in the initiation of obesity. Treatment starting on week 5 was designed to explore the function of VATMs in the progression of weight gain. The results show that intraperitoneal injection of clodronate liposomes effectively depleted VATMs, which blocked high-fat diet-induced weight gain, fat accumulation, insulin resistance, and hepatic steatosis. Similarly, clodronate liposomes suppressed progression of weight gain in mice after being fed with a high-fat diet for 4 weeks and improved insulin sensitivity. Gene expression analysis showed that depletion of VATMs was associated with downregulation of the expression of genes involved in lipogenesis and gluconeogenesis including acc1, fas, scd1, and pepck, decreased expression of genes involved in chronic inflammation including mcp1 and tnfα, and suppressed expression of macrophage specific marker genes of f4/80 and cd11c in adipose tissue. Depletion of VATMs was associated with prevention of the formation of crown-like structures in white adipose tissue and the maintenance of a low level of blood TNF-α. Collectively, these data demonstrate that VATMs appeared to play a crucial role in the development of obesity and obesity-associated diseases and suggest that adipose tissue macrophages could be regarded as a potential target for drug development in prevention and therapy of obesity and obesity-associated complications.






Similar content being viewed by others
REFERENCES
Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults 1999–2008. JAMA. 2010;303:235–41.
Moller DE, Kaufman KD. Metabolic syndrome: a clinical and molecular perspective. Annu Rev Med. 2005;56:45–62.
De Lauzon-Guillain B, Oliveira A, Monnery-Patris S. A review of methods to assess parental feeding practices and preschool children’s eating behavior: the need for further development of tools. J Acad Nutr Diet. 2012;112:1578–602.
Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–86.
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante Jr AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808.
Cancello R, Tordjman J, Poitou C, Guilhem G, Bouillot JL, Hugol D, et al. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes. 2006;55:1554–61.
Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30.
Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–8.
Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7.
Hirata A, Maeda N, Hiuge A, Hibuse T, Fujita K, Okada T, et al. Blockade of mineralocorticoid receptor reverses adipocyte dysfunction and insulin resistance in obese mice. Cardiovasc Res. 2009;84:164–72.
Ma Y, Liu D. Activation of pregnane X receptor by pregnenolone 16 α-carbonitrile prevents high-fat diet-induced obesity in AKR/J mice. PLoS One. 2012;7(6):e38734.
Milner JJ, Beck MA. The impact of obesity on the immune response to infection. Proc Nutr Soc. 2012;71:298–306.
Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker J, Vaddi K, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–24.
Van Rooijen N, Sanders A, van den Berg TK. Apoptosis of macrophages induced by liposome-mediated intracellular delivery of clodronate and propamidine. J Immunol Methods. 1996;193:93–9.
Lijnen HR. Angiogenesis and obesity. Cardiovasc Res. 2008;78:286–93.
Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–101.
Cao Y. Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nat Rev Drug Discov. 2010;9:107–15.
Leibovich SJ, Polverini PJ, Shepard HM, Wiseman DM, Shively V, Nuseir N. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature. 1997;329:630–2.
Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, et al. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res. 2000;6:3282–9.
Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, et al. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116:829–40.
Mariman EC, Wang P. Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell Mol Life Sci. 2010;67:1277–92.
Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29:1575–91.
Neyrinck AM, Cani PD, Dewulf EM, De Backer F, Bindels LB, Delzenne NM. Critical role of Kupffer cells in the management of diet-induced diabetes and obesity. Biochem Biophys Res Commun. 2009;385:351–6.
Huang W, Metlakunta A, Dedousis N, Zhang P, Sipula I, Dube JJ, et al. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes. 2010;59:347–57.
Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci USA. 2010;107:3441–6.
Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95–6.
Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–9.
Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91.
Feng B, Jiao P, Nie Y, Kim T, Jun D, van Rooijen N, et al. Clodronate liposomes improve metabolic profile and reduce visceral adipose macrophage content in diet-induced obese mice. PLoS One. 2011;6:e24358.
ACKNOWLEDGMENTS
The study was supported in part by grants from NIH (RO1EB007357 and RO1HL098295 to DL) and National Science Foundation in China (NSFC 81001572 and 81070238 to QS). We thank Miss Ryan Fugett for critical reading and English editing of the manuscript.
Conflict of interest
The authors claim no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bu, L., Gao, M., Qu, S. et al. Intraperitoneal Injection of Clodronate Liposomes Eliminates Visceral Adipose Macrophages and Blocks High-fat Diet-induced Weight Gain and Development of Insulin Resistance. AAPS J 15, 1001–1011 (2013). https://doi.org/10.1208/s12248-013-9501-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-013-9501-7