Abstract
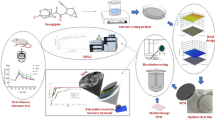
The hydration layer plays a key role in the controlled drug release of gel-forming matrix tablets. For poorly water-soluble drugs, matrix erosion is considered as the rate limiting step for drug release. However, few investigations have reported on the quantification of the relative importance of swelling and erosion in the release of poorly soluble drugs, and three-dimensional (3D) structures of the hydration layer are poorly understood. Here, we employed synchrotron radiation X-ray computed microtomography with 9-μm resolution to investigate the hydration dynamics and to quantify the relative importance of swelling and erosion on felodipine release by a statistical model. The 3D structures of the hydration layer were revealed by the reconstructed 3D rendering of tablets. Twenty-three structural parameters related to the volume, the surface area (SA), and the specific surface area (SSA) for the hydration layer and the tablet core were calculated. Three dominating parameters, including SA and SSA of the hydration layer (SA hydration layer and SSA hydration layer ) and SA of the glassy core (SA glassy core ), were identified to establish the statistical model. The significance order of independent variables was SA hydration layer > SSA hydration layer > SA glassy core , which quantitatively indicated that the release of felodipine was dominated by a combination of erosion and swelling. The 3D reconstruction and structural parameter calculation methods in our study, which are not available from conventional methods, are efficient tools to quantify the relative importance of swelling and erosion in the controlled release of poorly soluble drugs from a structural point of view.






Similar content being viewed by others
References
Tahara K, Yamamoto K, Toshiaki N. Overall mechanism behind matrix sustained release (SR) tablets prepared with hydroxypropyl methyl cellulose 2910. J Control Release. 1995;35:59–66.
Rodriguez CF, Bruneau N, Barra J, Alfonso D, Doelker E. Hydrophilic cellulose derivatives as drug delivery carriers: influence of substitution type on the properties of compressed matrix tablets. In: Wise DL, editor. Handbook of pharmaceutical controlled release technology. New York: CRC Press; 2000. p. 1–30.
Colombo P, Santi P, Bettini R, Brazel CS, Peppas NA. Drug release from swelling-controlled systems. In: Wise DL, editor. Handbook of pharmaceutical controlled release technology. New York: CRC Press; 2000. p. 183–209.
Lee PI. Modeling of drug release from matrix systems involving moving boundaries: approximate analytical solutions. Int J Pharm. 2011;418:18–27.
Lapidus H, Lordi NG. Some factors affecting the release of a water-soluble drug from a compressed hydrophilic matrix. J Pharm Sci. 1966;55:840–3.
Lapidus H, Lordi NG. Drug release from compressed hydrophilic matrices. J Pharm Sci. 1968;57:1292–301.
Peppas NA, Gurny R, Doelker E, Buri P. Modelling of drug diffusion through swellable polymeric systems. J Membr Sci. 1980;7:241–53.
Lee PI. Diffusional release of a solute from a polymeric matrix, approximate analytical solutions. J Membr Sci. 1980;7:255–75.
Lee PI, Peppas NA. Prediction of polymer dissolution in swellable controlled-release systems. J Control Release. 1987;6:207–15.
Harland RS, Gazzaniga A, Sangalli ME, Colombo P, Peppas NA. Drug/polymer matrix swelling and dissolution. Pharm Res. 1988;5:488–94.
Colombo P, Bettini R, Massimo G, Catellani PL, Santi P, Peppas NA. Drug diffusion front movement is important in drug release control from swellable matrix tablets. J Pharm Sci. 1995;84:991–7.
Tahara K, Yamamoto K, Nishihata T. Application of model-independent and model analysis for the investigation of effect of drug solubility on its release rate from hydroxypropyl methy cellulose sustained release tablets. Int J Pharm. 1996;133:17–27.
Bonferoni MC, Rossi S, Ferrari F, Bertoni M, Sinistri R, Caramella C. Characterization of three hydroxypropyl methy cellulose substitution types: rheological properties and dissolution behavior. Eur J Pharm Biopharm. 1995;41:242–6.
Gao P, Meury RH. Swelling of hydroxypropyl methylcellulose matrix tablets. 1. Characterization of swelling using a novel optical imaging method. J Pharm Sci. 1996;85:725–31.
Konrad R, Christ A, Zessin G, Cobet U. The use of ultrasound and penetrometer to characterize the advancement of swelling and eroding fronts in HPMC matrices. Int J Pharm. 1998;163:123–31.
Mikac U, Kristl J, Baumgartner S. Using quantitative magnetic resonance methods to understand better the gel-layer formation on polymer-matrix tablets. Drug Deliv. 2011;8:677–92.
Li HT, Gu XC. Correlation between drug dissolution and polymer hydration: a study using texture analysis. Int J Pharm. 2007;342:18–25.
Avalle P, Midwinter A, Gower N, Pygall SR. The use of near infrared spectroscopy to provide mechanistic insights into gel layer development in HPMC hydrophilic matrices. J Pharm Pharmacol. 2010;43:400–8.
Zhang Q, Gladden L, Avalle P, Mantle M. In vitro quantitative ((1))H and ((19))F nuclear magnetic resonance spectroscopy and imaging studies of Fluvastatin™ in Lescol® XL tablets in a USP-IV dissolution cell. J Control Release. 2011;156:345–54.
Hancock BC, Mullarney MP. X-ray microtomography of solid dosage forms. Pharm Technol. 2005;29:92–100.
Sinka IC, Burch SF, Tweed JH, Cunningham JC. Measurement of density variations in tablets using X-ray computed tomography. Int J Pharm. 2004;271:215–24.
Young PM, Nguyen K, Jones AS, Traini D. Microstructural analysis of porous composite materials: dynamic imaging of drug dissolution and diffusion through porous matrices. AAPS. 2008;10:560–4.
Tokudome Y, Ohshima H, Otsuka M. Non-invasive and rapid analysis for observation of internal structure of press-coated tablet using X-ray computed tomography. Drug Dev Ind Pharm. 2009;35:678–82.
Li HY, Yin XZ, Ji JQ, Sun LX, Shao Q, York P, et al. Microstructural investigation to the controlled release kinetics of monolith osmotic pump tablets via synchrotron radiation X-ray microtomography. Int J Pharm. 2012;427:270–5.
Laity PR, Cameron RE. Synchrotron X-ray microtomographic study of tablet swelling. Eur J Pharm Biopharm. 2010;75:263–76.
Abrahamsson B, Johansson D, Torstensson A, Wingstrand K. Evaluation of solubilizers in the drug release testing of hydrophilic matrix extended-release tablets of felodipine. Pharm Res. 1994;11:1093–7.
Shen F, Chen RC, Xiao TQ. GPU-based parallel computing for fast image reconstruction in micro CT. Nucl Tech. 2011;34:401–5.
Efentakis M, Pagoni I, Vlachou M, Avgoustakis K. Dimensional changes, gel layer evolution and drug release studies in hydrophilic matrices loaded with drugs of different solubility. Int J Pharm. 2007;339:66–75.
Ritger PL, Peppas NA. A simple equation for description of solute release. II Fickian and anomalous release from swellable devices. J Control Release. 1987;5:37–42.
Colombo P, Bettini R, Santi P, Peppas NA. Swellable matrices for controlled drug delivery: gel-layer behaviour, mechanisms and optimal performance. Pharm Sci Technol Today. 2000;3:198–204.
Reynolds TD, Mitchell SA, Balwinski KM. Investigation of the effect of tablet surface area/volume on drug release from hydroxypropylmethylcellulose controlled-release matrix tablets. Drug Dev Ind Pharm. 2002;28:457–66.
Bettini R, Catellani PL, Santi P, Massimo G, Peppas NA, Colombo P. Translocation of drug particles in HPMC matrix gel layer: effect of drug solubility and influence on release rate. J Control Release. 2001;70:383–91.
Acknowledgments
We are grateful for the beam time granted from the SSRF Key Program (Z12sr0040) and the financial support from the Ministry of Science and Technology of China (S2010GR0920, 2010ZX09401-402, and 2012ZX09301001-001), the Chinese Academy of Sciences Visiting Professorship for Senior International Scientists (Prof. Peter York) and the National Natural Science Foundation of China (81273453).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xianzhen Yin and Haiyan Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yin, X., Li, H., Guo, Z. et al. Quantification of Swelling and Erosion in the Controlled Release of a Poorly Water-Soluble Drug Using Synchrotron X-ray Computed Microtomography. AAPS J 15, 1025–1034 (2013). https://doi.org/10.1208/s12248-013-9498-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-013-9498-y