Abstract
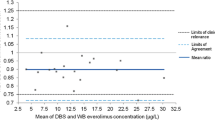
Dried blood spots (DBS) technology has been introduced as a microsampling alternative to traditional plasma or serum sampling for pharmacokinetics or toxicokinetics evaluation. The application of DBS has been established for many small molecule drugs at discovery, nonclinical, and clinical stages. However, the application of DBS for large molecule therapeutics development is not yet well-established. This article describes the method validation of a ligand binding assay (LBA) for DBS sampling of a therapeutic monoclonal antibody—AMG 162 (Denosumab). The original serum LBA was modified for the DBS method. A fit-for-purpose method validation was performed to evaluate accuracy and precision, selectivity, dilutional linearity, and stability. In addition, the parameters relevant to DBS, such as spot volume, extraction recovery, whole blood stability, and hematocrit effects, were evaluated. The validation results demonstrated assay robustness with inter-assay precision of ≤19%, inter-assay accuracy of ≤9%, and total error of ≤24%. Selectivity, extraction recovery, dilutional linearity, and stability were demonstrated. The validation results revealed some limitations of the possible effect of blood hematocrit on therapeutic concentration measurements and the caution required using whole blood for standards and quality controls preparation. This is the first article to describe a thorough method validation of an LBA using DBS for a therapeutic monoclonal antibody. The lessons learned can serve as a model process for future method validation of other LBAs for large molecule therapeutics or biomarkers using the DBS sampling method.






Similar content being viewed by others
REFERENCES
Edelbroek PM, Heijden J, Stolk LML. Dried blood spot methods in therapeutic drug monitoring: methods, assays, and pitfalls. Ther Drug Monit. 2009;31(3):327–36.
Tanna S, Lawson G. Analytical methods used in conjunction with dried blood spots. Anal Methods. 2011;3:1709–18.
Prince PJ, Matsuda KC, Retter MW, Scott G. Assessment of DBS technology for the detection of therapeutic antibodies. Bioanalysis. 2010;2(8):1449–60.
McDade TW, Williams S, Snodgrass JJ. What a drop can do: dried blood spots as a minimally invasive method for integrating biomarkers into population based research. Demography. 2007;44(4):899–925.
Guthrie R, Susi A. A simple phenylalanine method for detecting PKU in large populations of newborn infants. Pediatrics. 1963;32:338–43.
Beaudette P, Bateman KP. Discovery stage pharmacokinetics using dried blood spots. J Chromatogr B. 2004;809:153–8.
Clark GT, Haynes JJ, Bayliss MAJ, Burrows L. Utilization of DBS within drug discovery: development of a serial micro sampling pharmacokinetic study in mice. Bioanalysis. 2010;2:1477–88.
Evans CA. Dried blood spot analysis: a paradigm shift. AAPS Newsmagazine. April 2010.
Spooner N, Lad R, Barfield M. Dried blood spot as sample collection technique for the determination of pharmacokinetics in clinical studies: considerations for the validation of qualitative bioanalytical method. Anal Chem. 2009;81:1557–63.
Arnaud C. Technology renews a basic approach: dried blood spots offer advantages, but also challenges for pharmaceutical analysis. Chem Eng News. 2011;89(3):13–7.
Patel P, Mulla H, Tanna S, Pandya H. Facilitating pharmacokinetic studies in children: a new use of dried blood spots. Arch Dis Child. 2010;95:484–7.
Corran P, Cook J, Lynch C, et al. Dried blood spots as a source of anti-malarial antibodies for epidemiological studies. Malar J. 2008;7:195.
Solomon SS, Solomon S, Rodriguez I, et al. Dried blood spots: a valuable tool for HIV surveillance in developing/tropical countries. Int J STD AIDS. 2002;13:25–8.
Li W, Tse FLS. Dried blood spot sampling in combination with LC-MS/MS for quantitative analysis of small molecules. Biomed Chromatogr. 2010;24(1):49–65.
Emmons G, Rowland M. Pharmacokinetic considerations as to when to use dried blood spot sampling. Bioanalysis. 2010;2(11):1791–6.
Heinig K, Wirz T, Bucheli F, Gajate-Perez A. Determination of oseltamivir (Tamiflu®) and oseltamivir carboxylate in dried blood spots using offline or online extraction. Bioanalysis. 2011;3(4):421–37.
Rajendran S. Dried blood spot (DBS) assays for determination of biologics in whole blood. Oral presentation at the Mini Symposium Session—Dried Blood Spot Analysis for Biotherapeutics and Biomarkers (#190), 2011 AAPS—National Biotechnology Conference, San Francisco, CA, 17 May 2011.
Burns D, Rajendran S, Wang J, DeSilva B, Ma M. Validation feasibility of large molecules of differing modalities using dried blood spot samples. Poster presented at the 2011 AAPS—National Biotechnology Conference, San Francisco, CA, 17 May 2011.
Beharry M. DBS: a UK (MHRA) regulatory perspective. Bioanalysis. 2010;2(8):1363–4.
Davis CP. Hematocrit blood test. http://www.emedicinehealth.com/hematocrit_blood_test/article_em.htm. Accessed 13 July 2012.
FDA. Guidance for industry—bioanalytical methods validation. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), May 2001.
EMA Guideline on Validation of Bioanalytical Methods. Committee for Medicinal Products for Human Use, 1 Feb 2012.
Wang J, Lee JW, Burns D, Doherty D, Brunner L, Peterson M, DeSilva B. “Fit-for-purpose” method validation and application of a biomarker (C-terminal telopeptides of type 1 collagen) in denosumab clinical studies. AAPS J. 2009;11:385–94.
Blood Bank. Wikipedia, the free encyclopedia. http://en.wikipedia.org/wiki/Blood_bank. Accessed 13 Jul 2012.
Wang SS. What’s the shelf life of blood? Wall Street Journal. 1 December 2009. http://online.wsj.com/article/SB10001424052748703939404574567771148801570.html. Accessed 13 Jul 2012.
Mascheroni M. Extending the shelf life of donated blood. Innovation. 2008; 6(3). http://www.innovation-america.org/extending-shelf-life-donated-blood. Accessed 13 Jul 2012.
Timmerman P, White S, Globig S, Ludtke S, Brunet L, Smeraglia J. White paper/EBF recommendation on the validation of bioanalytical methods for dried blood spots. Bioanalysis. 2011;3(14):1567–75.
Denniff P, Spooner N. The effect of hematocrit on assay bias when using DBS samples for the quantitative bioanalysis of drugs. Bioanalysis. 2010;2(8):1385–95.
ACKNOWLEDGMENTS
We would like to thank Dr. Jean Lee and Dr. Theingi Thway for their contributions to the critical review of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burns, D., Brunner, L., Rajendran, S. et al. Validation of a Ligand Binding Assay Using Dried Blood Spot Sampling. AAPS J 15, 123–131 (2013). https://doi.org/10.1208/s12248-012-9430-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-012-9430-x