Abstract
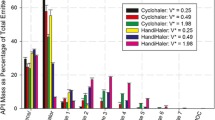
This study investigated the effect of modifying the design of the Cyclohaler on its aerosolization performance and comparability to the HandiHaler at multiple flow rates. The Cyclohaler and HandiHaler were designated as model test and reference unit-dose, capsule-based dry powder inhalers (DPIs), respectively. The flow field, pressure drop, and carrier particle trajectories within the Cyclohaler and HandiHaler were modeled via computational fluid dynamics (CFD). With the goal of achieving in vitro comparability to the HandiHaler, the CFD results were used to identify key device attributes and to design two modifications of the Cyclohaler (Mod 1 and Mod 2), which matched the specific resistance of the HandiHaler but exhibited different cyclonic flow conditions in the device. Aerosolization performance of the four DPI devices was evaluated by using the reference product's capsule and formulation (Spiriva capsule) and a multistage cascade impactor. The in vitro data showed that Mod 2 provided a closer match to the HandiHaler than the Cyclohaler and Mod 1 at 20, 39, and 55 l/min. The in vitro and CFD results together suggest that matching the resistance of test and reference DPI devices is not sufficient to attain comparable aerosolization performance, and the improved in vitro comparability of Mod 2 to the HandiHaler may be related to the greater degree of similarities of the flow rate of air through the pierced capsule (Qc) and the maximum impact velocity of representative carrier particles (Vn) in the Cyclohaler-based device. This investigation illustrates the importance of enhanced product understanding, in this case through the CFD modeling and in vitro characterization of aerosolization performance, to enable identification and modification of key design features of a test DPI device for achieving comparable aerosolization performance to the reference DPI device.







Similar content being viewed by others
REFERENCES
Oversteegen L, Rovini H, Belsey MJ. Respiratory drug market dynamics. Nat Rev Drug Discov. 2007;6:695–6.
Daley-Yates PT, Parkins DA. Establishing bioequivalence for inhaled drugs; weighing the evidence. Expert Opin Drug Deliv. 2011;8:1297–308.
Lee SL, Adams WP, Li BV, Conner DP, Chowdhury BA, Yu LX. In vitro considerations to support bioequivalence of locally acting drugs in dry powder inhalers for lung diseases. AAPS J. 2009;11:414–23.
Chodosh S, Flanders JS, Kesten S, Serby CW, Hochrainer D, Witek Jr TJ. Effective delivery of particles with the HandiHaler dry powder inhalation system over a range of chronic obstructive pulmonary disease severity. J Aerosol Med. 2001;14:309–15.
Telko MJ, Hickey AJ. Dry powder inhaler formulation. Respir Care. 2005;50:1209–27.
Newman S, Busse WW. Evolution of dry powder inhaler design, formulation, and performance. Respir Med. 2002;96:293–304.
Byron PR, Hindle M, Lange CF, Longest PW, McRobbie D, Oldham MJ, et al. In vivo–in vitro correlations: predicting pulmonary drug deposition from pharmaceutical aerosols. J Aerosol Med Pulm Drug Deliv. 2010;23 Suppl 2:59–69.
Xu Z, Mansour HM, Mulder T, McLean R, Langridge J, Hickey AJ. Dry powder aerosols generated by standardized entrainment tubes from drug blends with lactose monohydrate: 2. Ipratropium bromide monohydrate and fluticasone propionate. J Pharm Sci. 2010;99:3415–29.
Clark AR, Hollingworth AM. The relationship between powder inhaler resistance and peak inspiratory conditions in healthy volunteers—implications for in vitro testing. J Aerosol Med. 1993;6:99–110.
Coates MS, Chan H-K, Fletcher DF, Raper JA. Effect of design on the performance of a dry powder inhaler using computational fluid dynamics. Part 2: Air inlet size. J Pharm Sci. 2006;95:1382–92.
Coates MS, Fletcher DF, Chan H-K, Raper JA. Effect of design on the performance of a dry powder inhaler using computational fluid dynamics. Part 1: grid structure and mouthpiece length. J Pharm Sci. 2004;93:2863–76.
Coates MS, Chan H-K, Fletcher DF, Raper JA. Influence of air flow on the performance of a dry powder inhaler using computational and experimental analyses. Pharm Res. 2005;22:1445–53.
Coates MS, Fletcher DF, Chan H-K, Raper JA. The role of capsule on the performance of a dry powder inhaler using computational and experimental analyses. Pharm Res. 2005;22:923–32.
Inthavong K, Choi L-T, Tu J, Ding S, Thien F. Micron particle deposition in a tracheobronchial airway model under different breathing conditions. Med Eng Phys. 2010;32:1198–212.
Wong W, Fletcher DF, Traini D, Chan H-K, Crapper J, Young PM. Particle aerosolisation and break-up in dry powder inhalers 1: evaluation and modelling of venturi effects for agglomerated systems. Pharm Res. 2010;27:1367–76.
Wong W, Fletcher DF, Traini D, Chan H-K, Crapper J, Young PM. Particle aerosolisation and break-up in dry powder inhalers: evaluation and modelling of impaction effects for agglomerated systems. J Pharm Sci. 2011;100:2744–54.
Donovan MJ, Kim SH, Raman V, Smyth HD. Dry powder inhaler device influence on carrier particle performance. J Pharm Sci. 2012;101:1097–107.
Longest PW, Holbrook LT. In silico models of aerosol delivery to the respiratory tract—development and applications. Adv Drug Deliv Rev. 2012;64:296–311.
Longest PW, Hindle M. Condensational growth of combination drug-excipient submicrometer particles for targeted high efficiency pulmonary delivery: comparison of CFD predictions with experimental results. Pharm Res. 2012;29:707–21.
Longest PW, Tian G, Walenga RL, Hindle M. Comparing MDI and DPI aerosol deposition using in vitro experiments and a new stochastic individual path (SIP) model of the conducting airways. Pharm Res. 2012 (in press).
Criée CP, Meyer T, Petro W, Sommerer K, Zeising P. In vitro comparison of two delivery devices for administering formoterol: Foradil P and formoterol ratiopharm single-dose capsule inhaler. J Aerosol Sci. 2006;19:466–72.
Shih T-H, Liou WW, Shabbir A, Yang Z, Zhu J. A new kappa-epsilon eddy viscosity model for high Reynolds-number turbulent flows. Comput Fluids. 1995;24:227–38.
Launder BE, Rodi W. The turbulent wall jet measurements and modeling. Annu Rev Fluid Mech. 1983;15:429–59.
ANSYS. ANSYS Fluent 6.3 Users Guide. Lebanon, NH, USA. 2006. At: http://hpce.iitm.ac.in/website/Manuals/Fluent_6.3/Fluent.Inc/fluent6.3/help/index.htm. Accessed 4 Nov 2010.
Zhang Y, Finlay WH, Matida EA. Particle deposition measurements and numerical simulation in a highly idealized mouth-throat. J Aerosol Sci. 2004;35:789–803.
Liu Y, Matida EA, Gu J, Johnson MR. Numerical simulation of aerosol deposition in a 3-D human nasal cavity using RANS, RANS/EIM, and LES. J Aerosol Sci. 2007;38:683–700.
Morsi SA, Alexander AJ. An investigation of particle trajectories in two-phase flow systems. J Fluid Mech. 1972;55:193–208.
US Pharmacopeia. <601> Aerosols. Metered-dose inhalers and dry powder inhalers: particle size. Rockville: US Pharmacopeia 35/National Formulary 30, United States Pharmacopeial Convention; 2012.
Wachtel H, Ertunc O, Koksoy C, Delgado A. Aerodynamic optimization of Handihaler and Respimat: the roles of computational fluid dynamics and flow visualization. In: Dalby R, Byron P, Peart J, Suman J, Farr S, Young P, editors. Respiratory drug delivery 2008. Illinois: Davis Healthcare International Publishing; 2008. p. 165–74.
Nichols S, Wynn E. New approaches to optimizing dispersion in dry powder inhalers-dispersion force mapping and adhesion measurements. In: Dalby R, Byron P, Peart J, Suman J, Farr S, editors. Respiratory drug delivery 2008. Illinois: Davis Healthcare International Publishing; 2008. p. 175–84.
ACKNOWLEDGMENTS
The authors would like to thank Bhawana Saluja for her valuable comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
The opinions expressed in this paper by the authors do not necessarily reflect the views or policies of the Food and Drug Administration (FDA).
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Fig. 1
High-speed imaging of the capsule movement inside the HandiHaler upon exposure to the 39-l/min airflow (JPEG 21 kb)
Fig. 2
High-speed imaging of the capsule movement inside the Cyclohaler upon exposure to the 39-l/min airflow (JPEG 23 kb)
Rights and permissions
About this article
Cite this article
Shur, J., Lee, S., Adams, W. et al. Effect of Device Design on the In Vitro Performance and Comparability for Capsule-Based Dry Powder Inhalers. AAPS J 14, 667–676 (2012). https://doi.org/10.1208/s12248-012-9379-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-012-9379-9