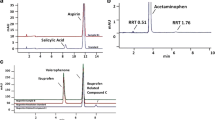
Abstract
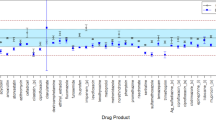
Efficacy and safety of medications used for the treatment of astronauts in space may be compromised by altered stability in space. We compared physical and chemical changes with time in 35 formulations contained in identical pharmaceutical kits stowed on the International Space Station (ISS) and on Earth. Active pharmaceutical content (API) was determined by ultra- and high-performance liquid chromatography after returning to Earth. After stowage for 28 months in space, six medications aboard the ISS and two of matching ground controls exhibited changes in physical variables; nine medications from the ISS and 17 from the ground met the United States Pharmacopeia (USP) acceptance criteria for API content after 28 months of storage. A higher percentage of medications from each flight kit had lower API content than the respective ground controls. The number of medications failing API requirement increased as a function of time in space, independent of expiration date. The rate of degradation was faster in space than on the ground for many of the medications, and most solid dosage forms met USP standard for dissolution after storage in space. Cumulative radiation dose was higher and increased with time in space, whereas temperature and humidity remained similar to those on the ground. Exposure to the chronic low dose of ionizing radiation aboard the spacecraft as well as repackaging of solid dosage forms in flight-specific dispensers may adversely affect stability of pharmaceuticals. Characterization of degradation profiles of unstable formulations and identification of chemical attributes of stability in space analog environments on Earth will facilitate development of space-hardy medications.







Similar content being viewed by others
REFERENCES
ICH. ICH quality guidelines. 2010. http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm065005.htm.
Lucas T, Bishara R, Seevers RH. A stability program for the distribution of drug products. Pharm Technol. 2004;2:68–73.
Chen XQ, Antman MD, Gesenberg C, Gudmundsson OS. Discovery pharmaceutics—challenges and opportunities. AAPS J. 2006;8(2):E402–8.
Carstensen JT. Stability of solids and solid dosage forms. J Pharm Sci. 1974;63(1):1–14.
Putcha L, Berens KL, Marshburn TH, Ortega HJ, Billica RD. Pharmaceutical use by U.S. astronauts on space shuttle missions. Aviat Space Environ Med. 1999;70(7):705–8.
Tietze KJ, Putcha L. Factors affecting drug bioavailability in space. J Clin Pharmacol. 1994;34(6):671–6.
Cosa G, Scaiano JC. Laser techniques in the study of drug photochemistry. Photochem Photobiol. 2004;80(2):159–74.
Langner MD, Maibach HI. Many common drugs in dermatology are light, temperature, or moisture-sensitive. Skin Therapy Lett. 2009;14(1):3–5.
Lyon RC, Taylor JS, Porter DA, Prasanna HR, Hussain AS. Stability profiles of drug products extended beyond labeled expiration dates. J Pharm Sci. 2006;95(7):1549–60.
Matsumoto M, Kojima K, Nagano H, Matsubara S, Yokota T. Photostability and biological activity of fluoroquinolones substituted at the 8 position after UV irradiation. Antimicrob Agents Chemother. 1992;36(8):1715–9.
Phillips G, Johnson BE, Ferguson J. The loss of antibiotic activity of ciprofloxacin by photodegradation. J Antimicrob Chemother. 1990;26(6):783–9.
Okeke CC, Srinivasan VS. Expiration and beyond-use dates. Am J Health Syst Pharm. 1998;55(5):433–4.
Okeke CC, Bailey L, Medwick T, Grady LT. Revised USP standards for product dating, packaging, and temperature monitoring. Am J Health Syst Pharm. 2000;57(15):1441–5.
Parks OW. Screening tests for sulfa drugs and/or dinitrobenzamide coccidiostats and their monoamino metabolites in chicken livers. J Assoc Off Anal Chem. 1985;68(1):20–3.
Allinson JG, Dansereau RJ, Sakr A. The effects of packaging on the stability of a moisture sensitive compound. Int J Pharm. 2001;221(1–2):49–56.
Van Weeren R, Sashidharan A. Sensitivity profiling of solid oral doses. Pharm Process. Apr 22 2008; 1–5.
Zwart SR, Kloeris VL, Perchonok MH, Braby L, Smith SM. Assessment of nutrient stability in foods from the space food system after long-duration space flight on the ISS. Food Sci. 2009;74(7):209–17.
Abramowicz M. Drugs past their expiration date. Med Lett. 2002;44(W1142B):93–4.
Patel H, Stalcup A, Dansereau R, Sakr A. The effect of excipients on the stability of levothyroxine sodium pentahydrate tablets. Int J Pharm. 2003;264(1–2):35–43.
Bosakova Z, Klouckova I, Tesarova E. Study of the stability of promethazine enantiomers by liquid chromatography using a vancomycin-bonded chiral stationary phase. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;770(1–2):63–9.
Moore TD, Horton R, Utrup LJ, Miller LA, Poupard JA. Stability of amoxicillin-clavulanate in BACTEC medium determined by high-performance liquid chromatography and bioassay. J Clin Microbiol. 1996;34(5):1321–2.
Vahdat L, Sunderland VB. Kinetics of amoxicillin and clavulanate degradation alone and in combination in aqueous solution under frozen conditions. Int J Pharm. 2007;342(1–2):95–104.
Scholtissek C, Webster RG. Long-term stability of the anti-influenza A compounds—amantadine and rimantadine. Antiviral Res. 1998;38(3):213–5.
Wu Y, Fassihi R. Stability of metronidazole, tetracycline HCl and famotidine alone and in combination. Int J Pharm. 2005;290(1–2):1–13.
Barbarina N, Tilquin B, de Hoffmann E. Radiosterilization of cefotaxime: investigation of potential degradation compounds by liquid chromatography-electrospray mass spectrometry. J Chromatogr A. 2001;929(1–2):51–61.
Maggi L, Segale L, Ochoa ME, Buttafava A, Faucitano A, Conte U. Chemical and physical stability of hydroxypropylmethylcellulose matrices containing diltiazem hydrochloride after gamma irradiation. J Pharm Sci. 2003;92(1):131–41.
Kane MP, Tsuji K. Radiolytic degradation scheme for 60Co-irradiated corticosteroids. J Pharm Sci. 1983;72(1):30–5.
Ahrabi SF, Sande SA, Waaler T, Graffner C. Effects of thermal neutron irradiation on some potential excipients for colonic delivery systems. Drug Dev Ind Pharm. 1999;25(4):453–62.
Benton ER, Benton EV. Space radiation dosimetry in low-Earth orbit and beyond. Nucl Instrum Methods Phys Res B. 2001;184(1–2):255–94.
National Council on Radiation Protection and Measurements (NCRP). Guidance on radiation received in space activities: report No. 98. Bethesda: NCRP; 1989.
ACKNOWLEDGMENTS
This research was funded by an NRA research grant from the National Aeronautics and Space Administration (NASA).
The authors acknowledge and appreciate the technical support provided by team members of the NASA Non-exercise Physiological Countermeasures Project and the International Space Station Medical Project. We thank Dr. Ramona Garza, a scientist from Universities Space Research Association, for providing reports of radiation dosimetry data and for her guidance on space radiation results for this study.
Author information
Authors and Affiliations
Corresponding author
ELECTRONIC SUPPLEMENTARY MATERIALS
Below is the link to the electronic supplementary material.
Table S I
(DOC 51 kb)
Table S II
(DOC 63 kb)
Table S III A
(DOC 66 kb)
Table S III B
(DOC 131 kb)
Rights and permissions
About this article
Cite this article
Du, B., Daniels, V.R., Vaksman, Z. et al. Evaluation of Physical and Chemical Changes in Pharmaceuticals Flown on Space Missions. AAPS J 13, 299–308 (2011). https://doi.org/10.1208/s12248-011-9270-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-011-9270-0