Abstract
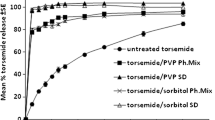
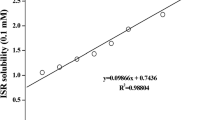
The purpose of the present investigation was to increase the solubility and dissolution rate of rofecoxib by the preparation of its solid dispersion with polyvinyl pyrrolidone K30 (PVP K30) using solvent evaporation method. Drug-polymer interactions were investigated using differential scanning calorimetry (DSC), x-ray diffraction (XRD), and Fourier transform infrared spectroscopy (FTIR). For the preparation of rofecoxib mouth dissolve tablets, its 1∶9 solid dispersion with PVP K30 was used with various disintegrants and sublimable materials. In an attempt to construct a statistical model for the prediction of disintegration time and percentage friability, a 32 randomized full and reduced factorial design was used to optimize the influence of the amounts of superdisintegrant and subliming agent. The obtained results showed that dispersion of the drug in the polymer considerably enhanced the dissolution rate. The drug-to-carrier ratio was the controlling factor for dissolution improvement. FTIR spectra revealed no chemical incompatibility between the drug and PVP K30. As indicated from XRD and DSC data, rofecoxib was in the amorphous form, which explains the better dissolution rate of the drug from its solid dispersions. Concerning the optimization study, the multiple regression analysis revealed that an optimum concentration of camphor and a higher percentage of crospovidone are required for obtaining rapidly disintegrating tablets. In conclusion, this investigation demonstrated the potential of experimental design in understanding the effect of the formulation variables on the quality of mouth dissolve tablets containing solid dispersion of a hydrophobic drug.
Similar content being viewed by others
References
Van den Mooter G, Wuyts M, Blaton N, et al. Physical stabilisation of amorphous ketoconazole in solid dispersions with polyvinylpyrrolidone K25. Eur J Pharm Sci. 2001; 12: 261–269.
Chiou WL, Riegelman S. Pharmaceutical applications of solid dispersions. J Pharm Sci. 1971; 60: 1281–1302.
Esnaashari S, Javadzadeh Y, Batchelor HK, Conway BR. The use of microviscometry to study polymer dissolution from solid dispersion drug delivery systems. Int J Pharm. 2005; 292: 227–230.
Van den Mooter G, Augustijns P, Blaton N, Kinget R. Physico-chemical characterization of solid dispersions of temazepam with polyethylene glycol 6000 and PVP K30. Int J Pharm. 1998; 164: 67–80.
Liu C, Desai KG. Characteristics of rofecoxib-polyethylene glycol 4000 solid dispersions and tablets based on solid dispersions. Pharm dev Technol. 2005; 10: 467–477.
Rawat S, Jain SK. Rofecoxib-beta-cyclodextrin inclusion complex for solubility enhancement. Pharmazie. 2003; 58: 639–641.
Masaki K. Orally disintegrating famotidine tablets. 22nd Conference on Pharmaceutical Technology; July 15–17, 1997; Kisarazu, Japan Tokyo, Japan: Academy of Pharmaceutical Science and Technology; 1997: 79–84.
Corveleyn S, Remon JP. Formulation and production of rapidly disintegrating tablets by lyophilization using hydrochlorothiazide as a model drug. Int J Pharm. 1997; 152: 215–225.
Roser BJ, Blair J, inventors. Rapidly soluble oral dosage forms, method of making some, and composition thereof. US patent 5 762 961. June 9, 1998.
Franco M, Trapani G, Latrofa A, et al. Dissolution properties and anticonvulsant activity of phenytoin-polyethylene glycol 6000 and-polyvinylpyrrolidone K-30 solid dispersions. Int J Pharm. 2001; 225: 63–73.
Arias MJ, Gins JM, Moyano JR, Rabasco AM. Influence of the preparation method of solid dispersions on their dissolution rate: study of triamterene-D-mannitol system. Int J Pharm. 1995; 123: 25–31.
Higuchi T, Connors KA. Phase-solubility techniques. Adv Anal Chem Instrum. 1965; 4: 117–210.
Sethia S, Squillante E. Solid dispersion of carbamazepine in PVP K30 by conventional solvent evaporation and supercritical methods. Int J Pharm. 2004; 272: 1–10.
Gohel M, Patel M, Amin A, Agrawal R, Dave R, Bariya N. Formulation design and optimization of mouth dissolve tablets of nimesulide using vacuum drying technique. AAPS PharmSciTech. 2004; 5:E36.
Seedher N, Bhatia S. Solubility enhancement of Cox-2 inhibitors using various solvent systems. AAPS PharmSciTech. 2003; 4: E33.
Abdul-Fattah AM, Bhargava HN. Preparation and in vitro evaluation of solid dispersions of halofantrine. Int J Pharm. 2002; 235: 17–33.
Torrado S, Torrado J, Cadorniga R. Preparation, dissolution and characterization of albendazole solid dispersions. Int J Pharm. 1996; 140: 247–250.
Torre P, Torrado S, Santiago T. Preparation, dissolution and characterization of praziquantel solid dispersions. Chem Pharm Bull (Tokyo). 1999; 47: 1629–1633.
Edward MR, ed. Oral solid dosage forms. In: Remington’s pharmaceutical Science. Easton, PA: Mack Publishing; 2000:858–893.
Koizumi K, Watanabe Y, Morita K, Utoguchi N, Matsumoto M. New method of preparing high-porosity rapidly saliva soluble compressed tablets using mannitol with camphor, a subliming material. Int J Pharm. 1997; 152: 127–131.
Mendenhall W, Sincich T, eds. Multiple regression. In: A Second Course in Business Statistics: Regression Analysis. 3rd ed. San Francisco, CA: Dellen Publishing Co; 1989: 141–226.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published: June 16, 2006
Rights and permissions
About this article
Cite this article
Sammour, O.A., Hammad, M.A., Megrab, N.A. et al. Formulation and optimization of mouth dissolve tablets containing rofecoxib solid dispersion. AAPS PharmSciTech 7, 55 (2006). https://doi.org/10.1208/pt070255
Received:
Accepted:
DOI: https://doi.org/10.1208/pt070255