Abstract
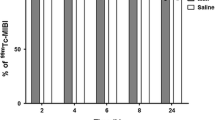
The present study investigates the preparation of celecoxib-loaded albumin microspheres and the biodistribution of technetium-99m (99mTc)-labeled celecoxib as well as its microspheres after intravenous administration. Microspheres were prepared using a natural polymer BSA using emulsification chemical cross-linking method. The prepared microspheres were characterized for entrapment efficiency, particle size, and in vitro drug release. Surface morphology was studied by scanning electron microscopy. Biodistribution studies were performed by radiolabeling celecoxib (CS) and its microspheres (CMS) using99mTc and injecting arthritic rats intravenously. The geometric mean diameter of the microspheres was found to be 5.46 μm. In vitro release studies indicated that the microspheres sustained the release of the drug for }6 days. Radioactivity measured in different organs after intravenous administration of celecoxib solution showed a significant amount of radioactivity in the liver and spleen. In case of celecoxib-loaded microspheres, a significant amount of radioactivity accumulated in the lungs. No significant difference (P>.1) in the radioactivity was observed between the inflamed joint and the noninflamed joint following intravenous injection of99mTc-CS. However, in case of the microspheres (CMS), the radioactivity present in the inflamed joint was 2.5-fold higher than in the noninflamed joint. The blood kinetic studies revealed that celecoxib-loaded albumin microspheres exhibited prolonged circulation than the celecoxib solution.
Similar content being viewed by others
References
Bogdansky S. Natural polymers as drug delivery systems. In: Chasin M, Langer R, eds.Biodegradable Polymers as Drug Delivery Systems. New York, NY: Marcel Dekker Inc; 1990:231–259.
Kramer PA. Albumin microspheres as vehicles for achieving specificity in drug delivery.J Pharm Sci. 1974;63:1646–1647.
Bernard NG, Shaw SM, Kessler WV, Landolt RR, Peck GE, Dockerty GH. Distribution and degradation of I-125 albumin microspheres and technetium 99m sulphur colloid.J Pharm Sci. 1980;15:30–34.
Schafer V, Briesen H, Rubsamen-Waigman H, Steffan AM, Royer C, Kreuter J. Phagocytosis and degradation of human serum albumin microspheres and nanoparticles in human macrophages.J Microencapsul. 1994;11:261–269.
Müller BG, Leuenberger H, Kissel T. Albumin nanospheres as carriers for passive drug targeting: an optimized manufacturing technique.Pharm Res. 1996;13:32–37.
Tomilson E, Burger JJ, Schoolderwoerd EMA, McVie JG. Human serum albumin microspheres for intra-arterial drug targeting of cytostatic compounds. In: Davis SS, Ilium JG, eds.Microspheres and Drug Therapy. Amsterdam, The Netherlands; Elsevier; 1984:75–91.
Tomilson E, Burger JJ. Monolithic albumin particles as drug carriers. In: Illum L, Davis SS, eds.Polymers in Controlled Drug Delivery. Bristol, UK: IOP Publishing Limited; 1987:25–48.
Wunder A, Muller-Ladner U, Stelzer EH, et al. Albumin-based drug delivery as novel therapeutic approach for rheumatoid arthritis.J Immunol. 2003;170:4793–4801.
Breton J, Pezzi N, Molinari A, et al. Prolonged half-life in the circulation of a chemical conjugate between a pro-urokinase derivative and human serum albumin.Eur J Biochem. 1995;231:563–569.
Srinath P, Chary MG, Vyas SP, Diwan PV. Long-circulating liposomes of indomethacin in arthritic rats: a biodisposition study.Pharm Acta Helv. 2000;74:399–404.
Metselaar JM, Wauben MH, Wagenaar-Hilbers JP, Boerman OC, StorM G. Complete remission of experimental arthritis by joint targeting of glucocorticoids with long-circulating liposomes.Arthritis Rheum. 2003;48:2059–2066.
Corvo ML, Boerman OC, Oyen WJ, et al. Intravenous administration of superoxide dismutase entrapped in long circulating liposomes. II. In vivo fate in a rat model of adjuvant arthritis.Biochim Biophys Acta. 1999;1419:325–334.
Perazella MA, Eras J. Are selective COX-2 inhibitors nephrotoxic?Am J Kidney Dis. 2000;35:937–940.
Bing RJ. Cyclooxygenase-2 inhibitors: is there an association with coronary or renal events?Curr Atheroscler Rep. 2003;5:114–117.
Brater DC. Effects of nonsteroidal anti-inflammatory drugs on renal function: focus on cyclooxygenase-2-selective inhibition.Am J Med 1999;107(6A):65S-70S; discussion 70S–71S.
Tacconelli S, Capone ML, Patrignani P. Clinical pharmacology of novel selective COX-2 inhibitors.Curr Pharm Des. 2004; 10:589–601.
Alper AB Jr, Meleg-Smith S, Krane NK. Nephrotic syndrome and interstitial nephritis associated with celecoxib.Am J Kidney Dis. 2002;40:1086–1090.
Lewis DA, Field WN, Hayes K, Alpar HO. The use of albumin microspheres in the treatment of carageenan-induced inflammation in the rat.J Pharm Pharmacol. 1992;44:271–274.
Alpar HO, Field WN, Hyde R, Lewis DA. The transport of microspheres from the gastrointestinal tract to inflammatory air pouches in the rat.J Pharm Pharmacol. 1989;41:194–196.
Tuncay M, Calis S, Kas HS, Ercan MT, Peksoy L, Hincal A. Diclofenac sodium incorporated PLGA (50:50) microspheres: formulation considerations and in-vitro/in-vivo evaluation.Int J Pharm. 2000;195(1–2):179–188.
Richardson VJ, Jeyashingh K, Jewkes RF. Properties of [99mTc] labeled liposomes in normal and tumor bearing rats.Biochem Soc Trans. 1977;5:290–291.
Theobald AE. Quality Control of Radiopharmaceuticals. In: Sampson CB, ed.Textbook of Radiopharmacy: Theory and Practice. New York, NY: Gordon and Breach Science Publishers; 1990:127–129.
Vogel GH, Vogel WH. Anti-athoratic and immunomodulatory activity. In:Drug Discovery and Evaluation-Pharmacological Assays. Berlin, Germany: Springer Verlag; 1997:421–460.
Wu MS, Robbins JC, Bugianesi RL, Ponpipom MM, Shen TV. Modified in vivo behavior of liposomes containing synthetic glycolipids.Biochim Biophys Acta. 1981;674:19–29.
Sahin S, Selek H, Ponchel G, et al. Preparation, characterization and in vivo distribution of terbutaline sulfate loaded albumin microspheres.J Control Release. 2002;82:345–358.
Jameela SR, Kumary TV, Lal AV, Jayakrishnan A. Progesterone loaded chitosan microspheres: a long acting biodegradable controlled delivery system.J Control Release. 1998;52:17–24.
United States Pharmacopeial Convention. Glutaral disinfectant solution. In:United States Pharmacopoeia XXII National Formulary XVII. Rockville, MD: United States Pharmacopeial Convention; 1990:1934.
Wunderlich G, Pinkert J, Andreeff M, et al. Preparation and biodistribution of rhenium-188 labeled albumin microspheres: a promising new agent for radiotherapy.Appl Radiat Isot. 2000;52:63–68.
Rhodes BA, Zölle I, Buchanan JW, Wagner HN. Radioactive albumin microspheres for studies of the pulmonary circulation.Radiology. 1969;92:1453–1460.
Lee TK, Sokoloski TD, Royer GP. Serum albumin beads: an injectable biodegradable system for the sustained release of drugs.Science. 1981;213(4504):233–235.
Komaki R, Liao Z, Milas L. Improvement strategies for molecular targeting: cyclo-oxygenase-2 inhibitors as radiosensitizers for non-small cell lung cancer.Semin Oncol. 2004;31:47–53.
Cuzzocrea S, Mazzon E, Sautebin L, et al. Protective effects of celecoxib in lung injury and red blood cells modification induced by carageenan in the rat.Biochem Pharmacol. 2002;63:785–795.
Cerchietti LC, Navigante AH, Peluffo GD, et al. Effects of celecoxib, medroxyprogesterone, and dietary intervention on systemic syndromes in patients with advanced lung adenocarcinoma: a pilot study.J Pain Symptom Manage. 2004;27:85–95.
Diperna CA, Bart RD, Sievers EM, Ma Y, Starnes VA, Bremner RM. Cyclooxygenase-2 inhibition decreases primary and metastatic tumor burden in a murine model of orthotopic lung adenocarcinoma.J Thorac Cardiovasc Surg. 2003;126:1129–1133.
Peluffo GD, Stillitani I, Rodriguez VA, Diament MJ, Klein SM. Reduction of tumor progression and paraneoplastic syndrome development in murine lung adenocarcinoma by nonsteroidal anti-inflammatory drugs.Int J Cancer. 2004;110:825–830.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thakkar, H., Sharma, R.K., Mishra, A.K. et al. Albumin microspheres as carriers for the antiarthritic drug celecoxib. AAPS PharmSciTech 6, 12 (2005). https://doi.org/10.1208/pt060112
Received:
Accepted:
DOI: https://doi.org/10.1208/pt060112