Abstract
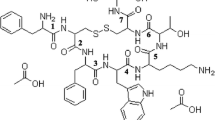
The purpose of this study was to prepare poly(ethylene glycol) (PEG)ylated octreotide and investigate the stability against acylation by polyester polymers such as poly(lactic acid) and poly(lactic-co-glycolic acid). Octreotide was modified by reaction with monomethoxy PEG-propionaldehyde (molecular weight 5,000) in the presence of sodium cyanoborohydride. The mono-PEGylated fraction was isolated by reverse-phase high-performance liquid chromatography (HPLC) and characterized by matrixassisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS). Circular dichroism demonstrated no significant secondary structural differences between mono-PEGylated octreotide (mono-PEG-octreotide) and intact octreotide. As a test system for the stability study against acylation reaction, lactic acid (LA) solutions with various concentrations and pH values were prepared with water dilution and subsequent accelerated equilibration at 90°C for 24 hours. Native octreotide was found to be acylated in all the diluted LA solutions with different concentrations (42.5%, 21.3%, and 8.5%, wt/wt) and pH values (2.25, 1.47, and 1.85, respectively). The remaining amounts of intact octreotide continuously decreased to 50% through 30 days of incubation at 37°C. MALDI-TOF MS identified the octreotide to be acylated by LA units. However, acylation reaction of mono-PEG-octreotide in LA solutions was negligible, and the remaining amounts of intact one through 30 days of incubation in LA solutions were also comparable to the initial concentration. These data suggest that mono-PEG-octreotide may prevent the acylation reaction in degrading PLA microspheres and possibly serve as a new source for somatostatin microsphere formulation.
Similar content being viewed by others
References
Van de Weert M, Hennink WE, Jiskoot W. Protein instability in poly(lactic-co-glycolic acid) microparticles. Pharm Res. 2000;17:1159–1167.
Fu K, Klibanov AM, Langer R. Protein stability in controlled-release systems. Nat Biotechnol. 2000;18:24–25.
Zhu G, Mallery SR, Schwendeman SP. Stabilization of proteins encapsulated in injectable poly (lactide-co-glycolide). Nat Biotechnol. 2000;18:52–57.
Shao PG, Bailey LC. Stabilization of pH-induced degradation of porcine insulin in biodegradable polyester microspheres. Pharm Dev Technol. 1999;4:633–642.
Rothen-Weinhold A, Oudry N, Schwach-Abdellaoui K, Frutiger-Hughes S, Hughes GJ, Jeannerat D, Burger U, Besseghir K, Gurny R. Formation of peptide impurities in polyester matrices during implant manufacturing. Eur J Pharm Biopharm. 2000;49:253–257.
Lucke A, Kiermaier J, Göpferich A. Peptide acylation by poly(a-hydroxy esters) Pharm Res. 2002;19:175–181.
Lucke A, Fustella E, Teßmar J, Gazzaniga A, Göpferich A. The effect of poly(ethylene glycol)-poly(D,L-lactic acid) diblock copolymers on peptide acylation. J Control Release. 2002;80:157–168.
Guerra PI, Acklin C, Kosky AA, Davis JM, Treuheit MJ, Brems DN. PEGylation prevents the N-terminal degradation of megakaryocyte growth and development factor. Pharm Res. 1998;15:1822–1827.
Lee KC, Tak KK, Park MO, Lee JT, Woo BH, Yoo SD, Lee HS, DeLuca, PP. Preparation and characterization of polyethylene glycol modified salmon calcitonins. Pharm Dev Technol. 1999;4:269–275.
Lee KC, Moon SC, Park MO, Lee JT, Na DH, Yoo SD, Lee HS, DeLuca PP. Isolation, characterization, and stability of positional isomers of mono-PEGylated salmon calcitonins. Pharm Res. 1999;16(6):813–818.
Hinds KD, Kim SW. Effects of PEG conjugation on insulin properties. Adv Drug Deliv Rev. 2002;54:505–530.
Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2:214–221.
Diwan M, Park TG. Pegylation enhances protein stability during encapsulation in PLGA microspheres. J Control Release. 2001;73:233–244.
Kim TH, Lee H, Park TG. Pegylated recombinant human epidermal growth factor (rhEGF) for sustained release from biodegradable PLGA microspheres. Biomaterials. 2002;23:2311–2317.
Diwan M, Park TG. Stabilization of recombinant interferon-alpha by pegylation for encapsulation in PLGA microspheres. Int J Pharm. 2003;252:111–122.
Na DH, Lee SD, Youn YS, et al. Monitoring of peptide acylation inside degrading PLGA microspheres by capillary electrophoresis and MALDI-TOF mass spectrometry. J Control Release. 2003;92:291–299.
Murty SB, Goodman J, Thanoo BC, DeLuca PP. Identification of chemically modified peptide from poly(D,L-lactide-co-glycolide) microspheres under in vitro release conditions. AAPS PharmSciTech. 2003;4(4): Article 50.
Lucke A, Göpferich A. Acylation of peptides by lactic acid solutions. Eur J Pharm Biopharm. 2003;55:27–33.
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85.
Kinstler OB, Brems DN, Lauren SL, Paige AG, Hamburger JB, Treuheit MJ. Characterization and stability of N-terminally PEGylated rhG-CSF. Pharm Res. 1996;13:996–1002.
Fu K, Pack DW, Klibanov AM, Langer R. Visual evidence of acidic environment within degrading poly(lactic-co-glycolic acid) (PLGA) microspheres. Pharm Res. 2000;17:100–106.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Na, D.H., Murty, S.B., Lee, K.C. et al. Preparation and stability of poly(ethylene glycol) (PEG)ylated octreotide for application to microsphere delivery. AAPS PharmSciTech 4, 72 (2003). https://doi.org/10.1208/pt040472
Received:
Accepted:
DOI: https://doi.org/10.1208/pt040472