Abstract
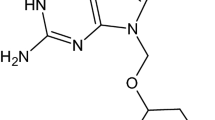
The purpose of this research is to investigate the sublimation process of DPC 963, a second-generation nonnucleoside reverse transcriptase inhibitor for HIV-1 retrovirus, and to better understant the effect of sublimation during active pharmaceutical ingredient (API) manufacture and formulation development, especially the drying processes. Sublimation of DPC 963 at 150°C and above was determined by thermogravimetric analysis-Fourier transform infrared (TGA-FTIR). The rates of sublimation at different temperatures were measured using isothermal TGA. Condensed material was collected and analyzed by differential scanning calorimetry (DSC), x-ray powder diffraction (XRPD), and infrared (IR) spectrometry. Benzoic acid was used as a reference standard to derive a linear logarithmic relationship between sublimation/evaporation rate and vapor pressure specific to the TGA system used in this study. Sublimation and evaporation of DPC 963 were found to follow apparent zero-order kinetics. Using the Eyring equation, the enthalpy and entropy of the sublimation and evaporation processes were obtained. The enthalpies of sublimation and evaporation were found to be 29 and 22 kcal/mol, respectively. The condensed material from the vapor phase was found to exist in 2 physical forms, amorphous and crystalline. Using benzoic acid as a reference standard, vapor pressure of DPC 963 at different temperatures was calculated using the linear logarithmic relationship obtained. DPC 963 undergoes sublimation at appreciable rates at 150°C and above but this is not likely to pose a serious issue during the manufacturing process. Vapor pressure estimation using thermogravimetric analysis provided sufficient accuracy to be used as a fast, simple, and safe alternative to the traditional methods of vapor pressure determination.
Similar content being viewed by others
References
Staszewski S, Morales-Ramires J, Tashima KT, et al. Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of HIV-2 infection in adults. N Engl J Med. 1999;341:1865–1973.
Adkin JC, Noble S. Efavirenz. Drug. 1998;56:1055–1064.
Corbett JW, Ko SS, Rodgers JD, et al. Expanded-spectrum nonnucleoside reverse transcriptase inhibitors inhibit clinically relevant mutant variants of human imunodeficiency virus type I. Antimicrob Agents Chemother. 1999;43:2893–2897.
Maurin MB, Dittert LW, Hussain AA. Therm ogravimetric analysis of ethylene-vinyl acetate copolymers with Fourier transform infrared analysis of the pyrolysis products. Thermochim Acta. 1991; 186: 97–102.
Gückel W, Kästel R, Kröhl T, Parg A. Methods for determining the vapor pressure of active ingredients used in crop protection. Part IV. An improved thermogravimetric determination based on evaporation rate. Pestic Sci. 1995;45:27–31.
TA Instruments Thermogravimetric Analysis 2950 users manual. New Castle, DE: TA Instruments, 1995.
Food and Drug Administration. USFDA Environmental Assessment Technical Guide. Rockville, MD: National Press Office, March 1987. No. 10.03.
Wiedemann HG. Application of thermogravimetry for vapor pressure determination. Thermochim Acta. 1972;3:355–366.
Elder JP. Sublimation measurements of pharmaceutical compounds by isothermal thermogravimetry. J Therm Anal. 1997;49:897–905.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xie, M., Ziemba, T.M. & Maurin, M.B. Sublimation characterization and vapor pressure estimation of an HIV nonnucleoside reverse transcriptase inhibitor using thermogravimetric analysis. AAPS PharmSciTech 4, 23 (2003). https://doi.org/10.1208/pt040223
Received:
Accepted:
DOI: https://doi.org/10.1208/pt040223