Abstract
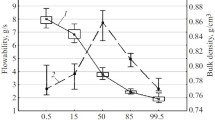
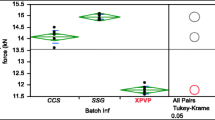
The aim of this paper was to study the effect of the granulate properties and tablet compression force on disintegrating force behavior in order to investigate the capability of the disintegrating force to characterize tablets that have the same composition but were manufactured in different conditions. Several tablets containing spironolactone in the external or internal granulated mixture of calcium carbonate and maize starch differing in particle size distribution, were prepared at 3 compression levels. The force developed by tablets during water uptake and disintegration was measured and plotted versus time. The curves obtained were analyzed by the Weibull equation in order to calculate the parameters characterizing the tablet disintegration kinetics. The disintegrating force time parameter, the maximum force developed, and the area under the curve were determined. In general, the reduction of time parameter value and/or the increase in maximum force developed corresponded to an acceleration in tablet disintegration. In addition, the area under the force curve increased in stronger tablets, monitoring in a sensitive way the tablet structural changes introduced by compression force. The results showed that the disintegrating force measurement can detect small changes in the structure of the tablet that cannot be discriminated by pharmacopoeia tests. The effect of manufacturing, in particular compression force, on tablet properties was quantified by the parameters of disintegrating force kinetics.
Similar content being viewed by others
References
Caramella C, Ferrari F, Bonferoni MC, Ronchi M. Disintegrants in solid dosage forms. Drug Dev Ind Pharm. 1990;16:2561–2577.
Ferrari F, Bertoni M, Bonferoni MC, et al. Influence of porosity and formula solubility of disintegrant efficiency in tablet. STP Pharma Sciences. 1995;5:116–121.
Vadas EB, Down GRB, Miller RA. Effect of compressional force on tablets containing cellulosic disintegrators, I: dimensionless disintegration values. J Pharm Sci. 1984;73:781–783.
Caramella C, Colombo P, Conte U, Gazzaniga A, La Manna A. The role of swelling in the disintegration process. International Journal of Pharmaceutical Technology & Product Manufacture 1984;5:1–5.
Colombo P, Conte U, Caramella C, Geddo M, La Manna A. Disintegrating force as a new formulation parameter. J Pharm Sci. 1984;73:701–705.
Catellani PL, Predella P, Bellotti A, Colombo P. Tablet water uptake and disintegration force measurements. Int J Pharm. 1989;51:63–66.
Massimo G, Catellani PL, Santi P, et al. Disintegration propensity of tablets evaluated by means of disintegrating force kinetics. Pharm Dev Technol. 2000;5:163–169.
Badwan AA, Nabulsi LN, Saleh MR, Arafat TA. Leveling off the effect of particle size on dissolution in spironolactone tablets. Congr Int Technol Pharm 5th. 1989;5:418–426.
ElShabouri MH. Nanoparticles for improving the dissolution and oral bioavailability of spironolactone, a poorly-soluble drug. STP Pharma Sciences. 2002;12:97–101.
Langenbucher L. Parametric representation of dissolution-rate curves by the RRSBW distribution. Die Pharmazeutische Industie. 1976;38:472–477.
Rodriguez F, Arama E, Maghi M, Paulin T, Ruffiac R. Application of the Weibull function to the study of in vivo/in vitro correlation. J Pharm Belg. 1990;45(3):173–183.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Massimo, G., Santi, P., Colombo, G. et al. The suitability of disintegrating force kinetics for studying the effect of manufacturing parameters on spironolactone tablet properties. AAPS PharmSciTech 4, 17 (2003). https://doi.org/10.1208/pt040217
Received:
Accepted:
DOI: https://doi.org/10.1208/pt040217