Abstract
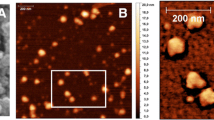
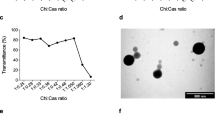
It was the purpose of this study to evaluate the potential of different molecular-weight chitosan-EDTA conjugates as a carrier matrix for nanoparticulate gene delivery systems. Covalent binding of EDTA to more than one chitosan chain provides a cross-linked polymer that is anticipated to produce stabilized particles. pDNA/chitosan-EDTA particles, generated via coazervation, were characterized in size and zeta potential by electrophoretic light scattering and electron microscopy. Stability was investigated at different pH values by enzymatic degradation and subsequent gel retardation assay. Lactate dehydrogenase assay was performed to determine toxicity. Furthermore, transfection efficiency into Caco-2 cells was assessed using a beta-galactosidase reporter gene. Chitosan-EDTA produced from low-viscous chitosan with 68% amino groups being modified by the covalent attachment of EDTA showed the highest complexing efficacy resulting in nanoparticles of 43 nm mean size and exhibiting a zeta potential of +6.3 mV. These particles were more stable at pH 8 than chitosan control particles. The cytotoxicity of chitosan-EDTA particles was below 1% over a time period of 4 hours. These new nanoplexes showed 35% improved in vitro transfection efficiency compared with unmodified chitosan nanoparticles. According to these results, the chitosan-EDTA conjugate may be a promising polymer for gene transfer.
Similar content being viewed by others
References
Patil SD, Rhodes DG, Burgess DJ. DNA-based therapeutics and DNA delivery systems: a comprehensive review. AAPS J. 2005;7:E61-E77.
Kay MA, Glorioso JC, Naldini L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat Med. 2001;7:33–40.
Timme TL, Hall SJ, Barrios R, Woo SL, Aguilar-Cordova E, Thompson TC. Local inflammatory response and vector spread after direct intraprostatic injection of a recombinant adenovirus containing the herpes simplex virus thymidine kinase gene and ganciclovir therapy in mice. Cancer Gene Ther. 1998;5:74–82.
Favre D, Provost N, Blouin V, et al. Immediate and long-term safety of recombinant adeno-associated virus injection into the nonhuman primate muscle. Mol Ther. 2001;4:559–566.
Luo D, Saltzman WM. Synthetic DNA delivery systems. Nat Biotechnol. 2000;18:33–37.
Audouy S, Molema G, de Leij L, Hoekstra D. Serum as a modulator of lipoplex-mediated gene transfection: dependence of amphiphile, cell type and complex stability. J Gene Med. 2000;2:465–476.
Borchard G. Chitosans for gene delivery. Adv Drug Deliv Rev. 2001;52:145–150.
Corsi K, Chellat F, Yahia L, Fernandes JC. Mesenchymal stem cells, MG63 and HEK293 transfection using chitosan-DNA nanoparticles. Biomaterials. 2003;24:1255–1264.
Haas J, Ravi Kumar MN, Borchard G, Bakowsky U, Lehr CM. Preparation and characterization of chitosan and trimethyl-chitosan-modified poly-(epsilon-caprolactone) nanoparticles as DNA carriers. AAPS PharmSciTech. 2005;6:E22-E30.
Mansouri S, Cuie Y, Winnik F, et al. Characterization of folatechitosan-DNA nanoparticles for gene therapy. Biomaterials. 2006;27:2060–2065.
Erbacher P, Zon S, Bettinger T, Steffan AM, Remy JS. Chitosanbased vector/DNA complexes for gene delivery: biophysical characteristics and transfection ability. Pharm Res. 1998;15: 1332–1339.
Park IK, Kim TH, Park YH, et al. Galactosylated chitosan-graft-poly (ethylene glycol) as hepatocyte-targeting DNA carrier. J Control Release. 2001;76:349–362.
Varga CM, Tedford NC, Thomas M, Klibanov AM, Griffith LG, Lauffenburger DA. Quantitative comparison of polyethylenimine formulations and adenoviral vectors in terms of intracellular gene delivery processes. Gene Ther. 2005;12:1023–1032.
Medberry P, Dennis S, Van Hecke T, DeLong RK. pDNA bioparticles: comparative heterogeneity, surface, binding, and activity analyses. Biochem Biophys Res Commun. 2004;319:426–432.
Bernkop-Schnurch A, Krajicek ME. Mucoadhesive polymers as platforms for peroral peptide delivery and absorption: synthesis and evaluation of different chitosan-EDTA conjugates. J Control Release. 1998;50:215–223.
Huang M, Khor E, Lim LY. Uptake and cytotoxicity of chitosan molecules and nanoparticles: effects of molecular weight and degree of deacetylation. Pharm Res. 2004;21:344–353.
Birnboim HC. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255.
Tang MX, Szoka FC. The influence of polymer structure on the interactions of cationic polymers with DNA and morphology of the resulting complexes. Gene Ther. 1997;4:823–832.
Huang M, Fong CW, Khor E, Lim LY. Transfection efficiency of chitosan vectors; effect of polymer molecular weight and degree of deacetylation. J Control Release. 2005;106:391–406.
Strand SP, Danielsen S, Christensen BE, Varum KM. Influence of chitosan structure on the formation and stability of DNA-chitosan polyelectrolyte complexes. Biomacromolecules. 2005;6:3357–3366.
Mislick KA, Baldeschwieler JD. Evidence for the role of proteoglycans in cation-mediated gene transfer. Proc Natl Acad Sci USA. 1996;93:12349–12354.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published: December 22, 2006
Rights and permissions
About this article
Cite this article
Loretz, B., Bernkop-Schnürch, A. In vitro evaluation of chitosan-EDTA conjugate polyplexes as a nanoparticulate gene delivery system. AAPS J 8, 85 (2006). https://doi.org/10.1208/aapsj080485
Received:
Accepted:
DOI: https://doi.org/10.1208/aapsj080485