Abstract
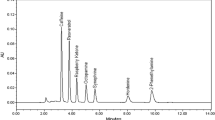
Six Australian and five overseas complementary medicines (CM) and meal replacement shake products were analysed for potential adulteration with two common active pharmaceutical ingredients, caffeine and sibutramine, using thin-layer chromatography and mass spectrometry. The declared amount of caffeine in each product was also reviewed. Finally, the products were examined for heavy metal contamination using inductively coupled plasma-mass spectrometry. The results showed that there was no detected adulteration of either caffeine (for those products that did not list caffeine as an ingredient) or sibutramine in the 11 products; however, based on the product labels, one Australian and one overseas (two in total) CM product contained more than the maximum daily safety limit (400 mg) of caffeine. Potentially excessive lead and/or chromium was detected in six products, including four Australian products and two products purchased online. One Australian CM product appeared to contain these heavy metals at concentrations at, or exceeding, the safety limits specified in the United States Pharmacopeia or set by the World Health Organization. The overconsumption of caffeine and heavy metals has the potential of causing significant health effects in consumers.


Similar content being viewed by others
References
Wright KL, Verney K, Brennan D, Lindsay D, Lindsay D, Smyth W. Administrative staff-self reported long-term conditions: findings from a regional Australian health service. Int J Workplace Health Manag. 2019;12(6):483–94.
Ee C, Cave AE, Naidoo D, Boyages J. Prevalence of and attitudes towards complementary therapy use for weight after breast cancer in Australia: a national survey. BMC Complement Altern Med. 2019;19(1):1–10.
Yoong SL, Carey ML, Sanson-Fisher RW, D’Este C. A cross-sectional study assessing the self-reported weight loss strategies used by adult Australian general practice patients. BMC Fam Pract. 2012;13(1):48.
Lauche R, Fuller NR, Cramer H, Wardle J, Sibbritt D, Adams J. Associations between complementary medicine, satisfaction with body weight and shape, and the use of methods to lose or control weight: results of a national survey of 8009 Australian women. Complement Ther Med. 2018;36:100–6.
Harvey K. Regulation of complementary medicines. Intern Med J. 2017;47(9):983–5.
Müller LS, Moreira APL, Muratt DT, Viana C, De Carvalho LM. An ultra-high performance liquid chromatography–electrospray tandem mass spectrometric method for screening and simultaneous determination of anorexic, anxiolytic, antidepressant, diuretic, laxative and stimulant drugs in dietary supplements marketed for weight loss. J Chromatogr Sci. 2019;57(6):528–40.
Hachem R, Assemat G, Martins N, Balayssac S, Gilard V, Martino R, et al. Proton NMR for detection, identification and quantification of adulterants in 160 herbal food supplements marketed for weight loss. J Pharm Biomed Anal. 2016;124:34–47.
Taing MW, Tan ETX, Williams GM, Clavarino AM, McGuire TM. Herbal and nutrient complementary medicines for weight loss: community pharmacists’ practices, attitudes, recommendations, information and education needs. Int J Pharm Pract. 2016;24(3):160–9.
Mathon C, Ankli A, Reich E, Bieri S, Christen P. Screening and determination of sibutramine in adulterated herbal slimming supplements by HPTLC-UV densitometry. Food Addit Contam Part A. 2014;31(1):15–20.
Khazan M, Hedayati M, Kobarfard F, Askari S, Azizi F. Identification and determination of synthetic pharmaceuticals as adulterants in eight common herbal weight loss supplements. Iran Red Crescent Med J. 2014;16(3):e15344.
Doménech-Carbóa A, Martinib M, de Carvalhob LM, Vianab C, Doménech-Carbóc MT, Silva M. Screening of pharmacologic adulterant classes in herbal formulations using voltammetry of microparticles. J Pharm Biomed Anal. 2013;74:194–204.
Trigueros L, Peña S, Ugidos A, Sayas-Barberá E, Pérez-Álvarez J, Sendra E. Food ingredients as anti-obesity agents: a review. Crit Rev Food Sci Nutr. 2013;53(9):929–42.
Tallett A, Blundell J, Rodgers R. Sibutramine & naloxone: infra-additive interaction in the regulation of appetite? Behav Brain Res. 2010;207(1):174–81.
James WPT, Caterson ID, Coutinho W, Finer N, Van Gaal LF, Maggioni AP, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med. 2010;363(10):905–17.
Gurley BJ, Steelman SC, Thomas SL. Multi-ingredient, caffeine-containing dietary supplements: history, safety, and efficacy. Clin Ther. 2015;37(2):275–301.
Viana C, Zemolin GM, Dal Molin TR, Gobo L, Ribeiro SM, Leal GC, et al. Detection and determination of undeclared synthetic caffeine in weight loss formulations using HPLC-DAD and UHPLC-MS/MS. J Pharm Anal. 2018;8(6):366–72.
Musgrave IF, Farrington RL, Hoban C, Byard RW. Caffeine toxicity in forensic practice: possible effects and under-appreciated sources. Forensic Sci Med Pathol. 2016;12(3):299–303.
Bessada SM, Alves RC, Oliveira MBP. Caffeine-based food supplements and beverages: trends of consumption for performance purposes and safety concerns. Food Res Int. 2018;109:310–9.
Neves DBDJ, Caldas ED. Determination of caffeine and identification of undeclared substances in dietary supplements and caffeine dietary exposure assessment. Food Chem Toxicol. 2017;105:194–202.
Pascali JP, Fais P, Vaiano F, Bertol E. Application of 404 HRAM screening and LC–MS/MS confirmation of active pharmaceutical ingredient in “natural” herbal supplements. Forensic Sci Int. 2018;286:e28–31.
Hoban CL, Musgrave IF, Coghlan ML, Power MW, Byard RW, Nash C, et al. Adulterants and contaminants in psychotropic herbal medicines detected with mass spectrometry and next409 generation DNA sequencing. Pharm Med. 2018;32(6):429–44.
Bolan S, Kunhikrishnan A, Seshadri B, Choppala G, Naidu R, Bolan NS, et al. Sources, distribution, bioavailability, toxicity, and risk assessment of heavy metal(loid)s in complementary medicines. Environ Int. 2017;108:103–18.
Flegal AR, Gallon C, Ganguli PM, Conaway CH. All the lead in China. Crit Rev Environ Sci Technol. 2013;43(17):1869–944.
Breeher L, Gerr F, Fuortes L. A case report of adult lead toxicity following use of Ayurvedic herbal medication. J Occup Med Toxicol. 2013;8(1):26.
Lin H, Chou S, Yang H, Hwang Y, Kuo C, Kao T, et al. Association of blood lead and mercury with estimated GFR in herbalists after the ban of herbs containing aristolochic acids in Taiwan. Occup Environ Med. 2013;70(8):545–51.
Filippini M, Baldisserotto A, Menotta S, Fedrizzi G, Rubini S, Gigliotti D, et al. Heavy metals and potential risks in edible seaweed on the market in Italy. Chemosphere. 2020;263:127983.
Gao Y, Li X, Dong J, Cao Y, Li T, Mielke HW. Snack foods and lead ingestion risks for school aged children: a comparative evaluation of potentially toxic metals and children’s exposure response of blood lead, copper and zinc levels. Chemosphere. 2020;261:127547.
Korfali SI, Hawi T, Mroueh M. Evaluation of heavy metals content in dietary supplements in Lebanon. Chem Cent J. 2013;7(1):10.
Abdulla NM, Adam B, Blair I, Oulhaj A. Heavy metal content of herbal health supplement products in Dubai–UAE: a cross-sectional study. BMC Complement Altern Med. 2019;19(1):276.
Sundar S, Chakravarty J. Antimony toxicity. Int J Environ Res Public Health. 2010;7(12):4267–77.
Sun H, Brocato J, Costa M. Oral chromium exposure and toxicity. Curr Environ Health Rep. 2015;2(3):295–303.
Shekhawat K, Chatterjee S, Joshi B. Chromium toxicity and its health hazards. Int J Adv Res. 2015;3(7):167–72.
Csupor D, Boros K, Dankó B, Veres K, Szendrei K, Hohmann J. Rapid identification of sibutramine in dietary supplements using a stepwise approach. Die Pharmazie. 2013;68(1):15–8.
Petkova-Gueorguieva E, Ivanov K, Gueorguiev S, Mihaylova A, Madzharov V, Ivanova S. Detection of sibutramine in herbal food supplements by UHPLC/HRMS and UHPLC/MS-MS. Biomed Res. 2018;29(14):3006–9.
Gray LJ, Cooper N, Dunkley A, Warren FC, Ara R, Abrams K, et al. A systematic review and mixed treatment comparison of pharmacological interventions for the treatment of obesity. Obes Rev. 2012;13(6):483–98.
Muschietti L, Redko F, Ulloa J. Adulteration of selected dietary supplements and their detection methods. Drug Test Anal. 2020;12(7):861–86.
Gu X, Jin Y, Dong F, Cai Y, You Z, You J, et al. Toward rapid analysis, forecast and discovery of bioactive compounds from herbs by jointly using thin layer chromatography and ratiometric surface-enhanced Raman spectroscopy technique. J Pharm Biomed Anal. 2018;153:9–15.
Phattanawasin P, Sotanaphun U, Sukwattanasinit T, Akkarawaranthorn J, Kitchaiya S. Quantitative determination of sibutramine in adulterated herbal slimming formulations by TLC image analysis method. Forensic Sci Int. 2012;219(1-3):96–100.
Therapeutic Goods Administration. High-moderate risk changes to permissible ingredients - Caffeine 2019 [Available from: https://www.tga.gov.au/high-moderate-risk-changes-permissible ingredients-caffeine.
Food Standards Australia New Zealand. Caffeine 2019 [Available from: https://www.foodstandards.gov.au/consumer/generalissues/Pages/Caffeine.aspx
British Pharmacopeia Commission. British Pharmacopoeia 2020 (Ph. Eur. 10.2). London2020.
Vincent JB. New evidence against chromium as an essential trace element. J Nutr. 2017;147(12):2212–9.
Milačič R, Ščančar J. Cr speciation in foodstuffs, biological 462 and environmental samples: methodological approaches and analytical challenges–a critical review. Trends Anal Chem. 2020;127:115888.
United States Pharmacopeia Convention. USP43-NF38. General Chapter <2232> Elemental Contaminants In Dietary Supplements. Rockville (MD)2020.
Bishop DP, Hare DJ, Clases D, Doble PA. Applications of liquid chromatography-inductively coupled plasma-mass spectrometry in the biosciences: a tutorial review and recent developments. Trends Anal Chem. 2018;104:11–21.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wong, P.H.B., Harnett, J.E., Clases, D. et al. An Analysis for Adulteration and Contamination of Over-the-Counter Weight-Loss Products. AAPS PharmSciTech 22, 78 (2021). https://doi.org/10.1208/s12249-021-01946-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-01946-7