Abstract
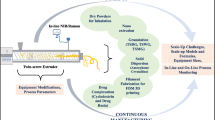
The hot melt extrusion (HME) technology was explored and optimized to solidify an amorphous nanosuspension using Quality by Design (QbD) methodology. A design of experiments (DoE) approach was used to perform a set of 15 experiments, varying independent variables (feed rate, input temperature, and screw speed) within a design space. Redispersibility index (RDI), moisture content, and process yield constituted the critical quality attributes (CQAs) of the experimental design. Regression analysis and ANOVA were employed to identify and estimate significant main effects and two-way interactions, and model the process of HME drying for predictive purposes. The optimized HME-dried end product was characterized for physicochemical properties using differential scanning calorimetry (DSC), X-ray powder diffractions (XRPD), polarized light microscopy (PLM), Fourier transform infrared spectroscopy (FTIR), and in vitro dissolution studies. The statistical analysis reveals feed rate and input temperature as significant independent variables, critically influencing RDI and moisture content of solidified end product. The model developed for process yield was insignificant at a p-value of 0.05. The API retained its amorphous nature after the extrusion process which was confirmed using DSC and XRPD techniques. PLM was unsuitable to differentiate and determine crystallinity of drug moiety in the presence of a semi-crystalline bulking agent, microcrystalline cellulose (MCC). In vitro dissolution study depicted solubility and dissolution enhancement for HME-dried amorphous nanosuspension in both the dissolution media which can be attributed to amorphous nature of nanosized drug particles. A well-designed study implemented by DoE aided in developing a robust and novel HME technique to dry aqueous nanosuspension.














Similar content being viewed by others
References
Pu X, Sun J, Li M, He Z. Formulation of nanosuspensions as a new approach for the delivery of poorly soluble drugs. Curr Nanosci. 2009;5(4):417–27.
Babu NJ, Nangia A. Solubility advantage of amorphous drugs and pharmaceutical cocrystals. Cryst Growth Des. 2011;11(7):2662–79.
Desai C, Prabhakar B. Nano-amorphous composites of cilostazol–HP-β-CD inclusion complexes: physicochemical characterization, structure elucidation, thermodynamic studies and in vitro evaluation. J Incl Phenom Macrocycl Chem. 2015;81(1–2):175–91.
Memişoğlu E, Bochot A, Özalp M, Şen M, Duchêne D, Hincal AA. Direct formation of nanospheres from amphiphilic β-cyclodextrin inclusion complexes. Pharm Res. 2003;20(1):117–25.
Patel AA. Liquid salt as green solvent: a novel eco-friendly technique to enhance solubility and stability of poorly soluble drugs: Long Island University, The Brooklyn Center. 2015.
Vogt M, Kunath K, Dressman JB. Dissolution enhancement of fenofibrate by micronization, cogrinding and spray-drying: comparison with commercial preparations. Eur J Pharm Biopharm. 2008;68(2):283–8.
Shah DA. To understand the thermodynamic and kinetic properties of nanocrystals using poorly soluble drugs: Long Island University, The Brooklyn Center. 2015.
Thakuria R, Delori A, Jones W, Lipert MP, Roy L, Rodríguez-Hornedo N. Pharmaceutical cocrystals and poorly soluble drugs. Int J Pharm. 2013;453(1):101–25.
Dugar RP, Gajera BY, Dave RH. Fusion method for solubility and dissolution rate enhancement of ibuprofen using block copolymer poloxamer 407. AAPS PharmSciTech. 2016;17(6):1428–40.
Tank D, Karan K, Gajera BY, Dave RH. Investigate the effect of solvents on wet granulation of microcrystalline cellulose using hydroxypropyl methylcellulose as a binder and evaluation of rheological and thermal characteristics of granules. Saudi Pharm J. 2018;26(4):593–602.
Brough C, Williams Iii R. Amorphous solid dispersions and nano-crystal technologies for poorly water-soluble drug delivery. Int J Pharm. 2013;453(1):157–66.
Murdande SB, Pikal MJ, Shanker RM, Bogner RH. Solubility advantage of amorphous pharmaceuticals: I. A thermodynamic analysis. J Pharm Sci. 2010;99(3):1254–64.
Bates S, Zografi G, Engers D, Morris K, Crowley K, Newman A. Analysis of amorphous and nanocrystalline solids from their X-ray diffraction patterns. Pharm Res. 2006;23(10):2333–49.
Hancock BC, Zografi G. Characteristics and significance of the amorphous state in pharmaceutical systems. J Pharm Sci. 1997;86(1):1–12.
Laitinen R, Löbmann K, Strachan CJ, Grohganz H, Rades T. Emerging trends in the stabilization of amorphous drugs. Int J Pharm. 2013;453(1):65–79.
Laitinen R, Löbmann K, Grohganz H, Priemel P, Strachan CJ, Rades T. Supersaturating drug delivery systems: the potential of co-amorphous drug formulations. Int J Pharm. 2017;532:1–12.
Baghel S, Cathcart H, O'Reilly NJ. Polymeric amorphous solid dispersions: a review of amorphization, crystallization, stabilization, solid-state characterization, and aqueous solubilization of biopharmaceutical classification system class II drugs. J Pharm Sci. 2016;105(9):2527–44.
Chaudhari SP, Dave RH. Evaluating the effects of different molecular weights of polymers in stabilizing supersaturated drug solutions and formulations using various methodologies of the model drug: fenofibrate. J Pharm Sci Pharmacol. 2015;2(3):259–76.
Ruddy SB, Eickhoff WM, Liversidge G. Site-specific adhesion within the GI tract using nanoparticles stabilized by high molecular weight, linear poly (ethylene oxide) polymers. Google Patents. 1996.
Mittal G, Sahana D, Bhardwaj V, Kumar MR. Estradiol loaded PLGA nanoparticles for oral administration: effect of polymer molecular weight and copolymer composition on release behavior in vitro and in vivo. J Control Release. 2007;119(1):77–85.
Mostafa AA, Zaazou MH, Chow LC, Mahmoud AA, Zaki DY, Basha M, et al. Injectable nanoamorphous calcium phosphate based in situ gel systems for the treatment of periapical lesions. Biomed Mater. 2015;10(6):065006.
R Serrano D, H Gallagher K, Marie Healy A. Emerging nanonisation technologies: tailoring crystalline versus amorphous nanomaterials. Curr Top Med Chem. 2015;15(22):2327–40.
Jog R, Kumar S, Shen J, Jugade N, Tan DCT, Gokhale R, et al. Formulation design and evaluation of amorphous ABT-102 nanoparticles. Int J Pharm. 2016;498(1):153–69.
Kumar S, Shen J, Burgess DJ. Nano-amorphous spray dried powder to improve oral bioavailability of itraconazole. J Control Release. 2014;192:95–102.
Yamasaki K, Kwok PCL, Fukushige K, Prud’homme RK, Chan H-K. Enhanced dissolution of inhalable cyclosporine nano-matrix particles with mannitol as matrix former. Int J Pharm. 2011;420(1):34–42.
Abdelwahed W, Degobert G, Stainmesse S, Fessi H. Freeze-drying of nanoparticles: formulation, process and storage considerations. Adv Drug Deliv Rev. 2006;58(15):1688–713.
Bohr A, Water J, Beck-Broichsitter M, Yang M. Nanoembedded microparticles for stabilization and delivery of drug-loaded nanoparticles. Curr Pharm Des. 2015;21(40):5829–44.
Kumar S, Gokhale R, Burgess DJ. Sugars as bulking agents to prevent nano-crystal aggregation during spray or freeze-drying. Int J Pharm. 2014;471(1–2):303–11.
Cerdeira AM, Mazzotti M, Gander B. Formulation and drying of miconazole and itraconazole nanosuspensions. Int J Pharm. 2013;443(1–2):209–20.
Dan J, Ma Y, Yue P, Xie Y, Zheng Q, Hu P, et al. Microcrystalline cellulose-carboxymethyl cellulose sodium as an effective dispersant for drug nanocrystals: a case study. Carbohydr Polym. 2016;136:499–506.
Sofie V, Jan V, Ludo F, Patrick A. Microcrystalline cellulose, a useful alternative for sucrose as a matrix former during freeze-drying of drug nanosuspensions–a case study with itraconazole. Eur J Pharm Biopharm. 2008;70(2):590–6.
Maniruzzaman M, Nokhodchi A. Continuous manufacturing via hot-melt extrusion and scale up: regulatory matters. Drug Discov Today. 2017;22(2):340–51.
Baumgartner R, Eitzlmayr A, Matsko N, Tetyczka C, Khinast J, Roblegg E. Nano-extrusion: a promising tool for continuous manufacturing of solid nano-formulations. Int J Pharm. 2014;477(1):1–11.
Hao J, Fang X, Zhou Y, Wang J, Guo F, Li F, et al. Development and optimization of solid lipid nanoparticle formulation for ophthalmic delivery of chloramphenicol using a Box-Behnken design. Int J Nanomedicine. 2011;6:683.
Sharma D, Maheshwari D, Philip G, Rana R, Bhatia S, Singh M, et al. Formulation and optimization of polymeric nanoparticles for intranasal delivery of lorazepam using Box-Behnken design: in vitro and in vivo evaluation. Biomed Res Int. 2014;2014:1–14.
Nekkanti V, Muniyappan T, Karatgi P, Hari MS, Marella S, Pillai R. Spray-drying process optimization for manufacture of drug–cyclodextrin complex powder using design of experiments. Drug Dev Ind Pharm. 2009;35(10):1219–29.
Mishra B, Sahoo J, Dixit PK. Enhanced bioavailability of cinnarizine nanosuspensions by particle size engineering: optimization and physicochemical investigations. Mater Sci Eng C. 2016;63:62–9.
Thakkar HP, Patel BV, Thakkar SP. Development and characterization of nanosuspensions of olmesartan medoxomil for bioavailability enhancement. J Pharm Bioallied Sci. 2011;3(3):426–34.
Zhang X, Liu J, Qiao H, Liu H, Ni J, Zhang W, et al. Formulation optimization of dihydroartemisinin nanostructured lipid carrier using response surface methodology. Powder Technol. 2010;197(1–2):120–8.
Talekar SD, Dave RH. Solubility enhancement of a BCS class II drug using granulated fumed silica as an adsorbent. J Pharm Res 2017;18(6):1–15. https://doi.org/10.9734/JPRI/2017/36872.
Gajera BY, Dugar RP, Dave RH. Formulation development and optimization of ibuprofen poloxamer melt granules using hydrophilic excipients. Br J Pharm Res. 2016;13(6):1–19.
Lu Y, Yu Y, Tang X. Sucrose acetate isobutyrate as an in situ forming system for sustained risperidone release. J Pharm Sci. 2007;96(12):3252–62.
Crowley MM, Zhang F, Repka MA, Thumma S, Upadhye SB, Kumar Battu S, et al. Pharmaceutical applications of hot-melt extrusion: part I. Drug Dev Ind Pharm. 2007;33(9):909–26.
Quek SY, Chok NK, Swedlund P. The physicochemical properties of spray-dried watermelon powders. Chem Eng Process Process Intensif. 2007;46(5):386–92.
Madgulkar A, Bandivadekar M, Shid T, Rao S. Sugars as solid dispersion carrier to improve solubility and dissolution of the BCS class II drug: clotrimazole. Drug Dev Ind Pharm. 2016;42(1):28–38.
Karolewicz B, Gajda M, Owczarek A, Pluta J, Górniak A. Physicochemical characterization and dissolution studies of solid dispersions of clotrimazole with Pluronic F127. Trop J Pharm Res. 2014;13(8):1225–32.
Pachuau L, Vanlalfakawma DC, Tripathi SK, Lalhlenmawia H. Muli bamboo (Melocanna baccifera) as a new source of microcrystalline cellulose. 2014.
Mitra A, Kesisoglou F. Impaired drug absorption due to high stomach pH: a review of strategies for mitigation of such effect to enable pharmaceutical product development. Mol Pharm. 2013;10(11):3970–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gajera, B.Y., Shah, D.A. & Dave, R.H. Investigating a Novel Hot Melt Extrusion-Based Drying Technique to Solidify an Amorphous Nanosuspension Using Design of Experiment Methodology. AAPS PharmSciTech 19, 3778–3790 (2018). https://doi.org/10.1208/s12249-018-1189-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-018-1189-7