Abstract
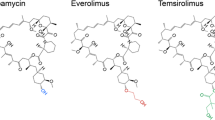
Rapamycin is commonly used in chemotherapy and posttransplantation rejection suppression, where sustained release is preferred. Conventionally, rapamycin has to be administered in excess due to its poor solubility, and this often leads to cytotoxicity and undesirable side effects. In addition, rapamycin has been shown to be hydrolytically unstable, losing its bioactivity within a few hours. The use of drug delivery systems is hypothesized to preserve the bioactivity of rapamycin, while providing controlled release of this otherwise potent drug. This paper reports on the use of microparticles (MP) as a means to tune and sustain the delivery of bioactive rapamycin for up to 30 days. Rapamycin was encapsulated (100% efficiency) in poly(lactic-co-glycolic acid) (PLGA), polycaprolactone (PCL), or a mixture of both via an emulsion method. The use of different polymer types and mixture was shown to achieve a variety of release kinetics and profile. Released rapamycin was subsequently evaluated against breast cancer cell (MCF-7) and human lymphocyte cell (Jurkat). Inhibition of cell proliferation was in good agreement with in vitro release profiles, which confirmed the intact bioactivity of rapamycin. For Jurkat cells, the suppression of cell growth was proven to be effective up to 20 days, a duration significantly longer than free rapamycin. Taken together, these results demonstrate the ability to tune, sustain, and preserve the bioactivity of rapamycin using MP formulations. The sustained delivery of rapamycin could lead to better therapeutic effects than bolus dosage, at the same time improving patient compliance due to its long-acting duration.





Similar content being viewed by others
References
Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2013. In: National vital. Statistics reports. Volume 61 NM, 2013. Deaths: final data for 2010, editor. Centers for Disease Control and Prevention; 2013.
Jhunjhunwala S, Balmert SC, Raimondi G, Dons E, Nichols EE, Thomson AW, et al. Controlled release formulations of IL-2, TGF-beta1 and rapamycin for the induction of regulatory T cells. J Control Release. 2012;159(1):78–84.
Brown DM. Drug delivery systems in cancer therapy. Totowa: Humana; 2004.
Baker JR, Jr. Dendrimer-based nanoparticles for cancer therapy. Hematol/Educ Prog Am Soc Hematol Am Soc Hematol Educ Prog. 2009:708–19.
Abouelfadel Z, Crawford ED. Leuprorelin depot injection: patient considerations in the management of prostatic cancer. Ther Clin Risk Manag. 2008;4(2):513–26.
Wicki A, Witzigmann D, Balasubramanian V, Huwyler J. Nanomedicine in cancer therapy: challenges, opportunities, and clinical applications. J Control Release. 2015;200:138–57.
Lee WL, Loo SCJ. Revolutionizing drug delivery through biodegradable multilayered particles. J Drug Target. 2012;20(8):633–47.
Lee WL, Widjaja E, Loo SCJ. One-step fabrication of triple-layered polymeric microparticles with layer localization of drugs as a novel drug-delivery system. Small. 2010;6(9):1003–11.
Lee WL, Seh YC, Widjaja E, Chong HC, Tan NS, Loo SC. Fabrication and drug release study of double-layered microparticles of various sizes. J Pharm Sci. 2012;101(8):2787–97.
Singh MN, Hemant KSY, Ram M, Shivakumar HG. Microencapsulation: a promising technique for controlled drug delivery. Res Pharm Sci. 2010;5(2):65–77.
Lee WL, Loei C, Widjaja E, Loo SCJ. Altering the drug release profiles of double-layered ternary-phase microparticles. J Control Release. 2011;151(3):229–38.
Lim MPA, Lee WL, Widjaja E, Loo SCJ. One-step fabrication of core-shell structured alginate-PLGA/PLLA microparticles as a novel drug delivery system for water soluble drugs. Biomater Sci. 2013;1(5):486–93.
Li J, Kim SG, Blenis J. Rapamycin: one drug, many effects. Cell Metab. 2014;19(3):373–9.
Law BK. Rapamycin: an anti-cancer immunosuppressant? Crit Rev Oncol/Hematol. 2005;56(1):47–60.
Saunders RN, Metcalfe MS, Nicholson ML. Rapamycin in transplantation: a review of the evidence. Kidney Int. 2001;59(1):3–16.
Malaguti P, Vari S, Cognetti F, Fabi A. The mammalian target of rapamycin inhibitors in breast cancer: current evidence and future directions. Anticancer Res. 2013;33(1):21–8.
Seto B. Rapamycin and mTOR: a serendipitous discovery and implications for breast cancer. Clin Transl Med. 2012;1(1):29.
Luan FL, Ding R, Sharma VK, Chon WJ, Lagman M, Suthanthiran M. Rapamycin is an effective inhibitor of human renal cancer metastasis. Kidney Int. 2003;63(3):917–26.
Cifarelli V, Lashinger LM, Devlin KL, Dunlap SM, Huang J, Kaaks R, et al. Metformin and rapamycin reduce pancreatic cancer growth in obese prediabetic mice by distinct microRNA-regulated mechanisms. Diabetes. 2015;64(5):1632–42.
Battelli C, Cho DC. mTOR inhibitors in renal cell carcinoma. Therapy. 2011;8(4):359–67.
Dai ZJ, Gao J, Ma XB, Kang HF, Wang BF, Lu WF, et al. Antitumor effects of rapamycin in pancreatic cancer cells by inducing apoptosis and autophagy. Int J Mol Sci. 2012;14(1):273–85.
Simamora P, Alvarez JM, Yalkowsky SH. Solubilization of rapamycin. Int J Pharm. 2001;213(1–2):25–9.
Abraham RT, Gibbons JJ, Graziani EI. Chapter 17—chemistry and pharmacology of rapamycin and its derivatives. In: Michael NH, Fuyuhiko T, editors. The enzymes, vol. 27. San Diego: Academic; 2010. p. 329–66.
CV PP. Rapamune® (sirolimus) Oral solution and tablets 1999. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/021083s058,021110s075lbl.pdf.
Limited P. Rapamune summary of product characteristics https://www.medicines.org.uk/emc/medicine/5747: eMC; 27 Oct 2015 [updated 27 Oct 2015; cited 2016 19 Feb].
Ferron GM, Jusko WJ. Species differences in sirolimus stability in humans, rabbits, and rats. Drug Metab Dispos. 1998;26:83–4.
Streit F, Christians U, Schiebel HM, Napoli KL, Ernst L, Linck A, et al. Sensitive and specific quantification of sirolimus (rapamycin) and its metabolites in blood of kidney graft recipients by HPLC/electrospray-mass spectrometry. Clin Chem. 1996;42(9):1417–25.
Oyler AR, Segmuller BE, Sun Y, Polshyna A, Dunphy R, Armstrong BL, et al. Forced degradation studies of rapamycin: identification of autoxidation products. J Pharm Biomed Anal. 2012;59:194–200.
Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9(5):324–37.
Holt DA, Konialian AL, Brandt M, Levy MA, Bossard MJ, Luengo JI, et al. Structure-activity studies of nonmacrocyclic rapamycin derivatives. Bioorg Med Chem Lett. 1993;3(10):1977–80.
Varde NK, Pack DW. Microspheres for controlled release drug delivery. Expert Opin Biol Ther. 2004;4(1):35–51.
Fahr A, Liu X. Drug delivery strategies for poorly water-soluble drugs. Expert Opin Drug Deliv. 2007;4(4):403–16.
Tyler B, Wadsworth S, Recinos V, Mehta V, Vellimana A, Li K, et al. Local delivery of rapamycin: a toxicity and efficacy study in an experimental malignant glioma model in rats. Neuro-Oncology. 2011;13(7):700–9.
Nanjwade KB, Patel JD, Parikh AK, Nanjwade KV, Manvi FV. Development and characterization of solid-lipid microparticles of highly insoluble drug sirolimus. J Bioequivalence Bioavailab. 2011;03(01).
Shah M, Edman MC, Janga SR, Shi P, Dhandhukia J, Liu SY, et al. A rapamycin-binding protein polymer nanoparticle shows potent therapeutic activity in suppressing autoimmune dacryoadenitis in a mouse model of Sjogren’s syndrome. J Control Release. 2013;171(3):269–79.
Kauffman KJ, Kanthamneni N, Meenach SA, Pierson BC, Bachelder EM, Ainslie KM. Optimization of rapamycin-loaded acetalated dextran microparticles for immunosuppression. Int J Pharm. 2012;422(1–2):356–63.
Jhunjhunwala S, Raimondi G, Thomson AW, Little SR. Delivery of rapamycin to dendritic cells using degradable microparticles. J Control Release. 2009;133(3):191–7.
Leong DT, Ng KW. Probing the relevance of 3D cancer models in nanomedicine research. Adv Drug Deliv Rev. 2014;79–80:95–106.
Pillai O, Panchagnula R. Polymers in drug delivery. Curr Opin Chem Biol. 2001;5(4):447–51.
Dinarvand R, Sepehri N, Manoochehri S, Rouhani H, Atyabi F. Polylactide-co-glycolide nanoparticles for controlled delivery of anticancer agents. Int J Nanomedicine. 2011;6:877–95.
Hines DJ, Kaplan DL. Poly(lactic-co-glycolic) acid-controlled-release systems: experimental and modeling insights. Crit Rev Ther Drug Carrier Syst. 2013;30(3):257–76.
Hamaguchi T, Matsumura Y, Suzuki M, Shimizu K, Goda R, Nakamura I, et al. NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paclitaxel. Br J Cancer. 2005;92(7):1240–6.
Khoee S, Hassanzadeh S, Goliaie B. Effects of hydrophobic drug-polyesteric core interactions on drug loading and release properties of poly(ethylene glycol)-polyester-poly(ethylene glycol) triblock core-shell nanoparticles. Nanotechnology. 2007;18(17):175602.
Rothstein SN, Little SR. A “tool box” for rational design of degradable controlled release formulations. J Mater Chem. 2011;21(1):29–39.
Fredenberg S, Wahlgren M, Reslow M, Axelsson A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—a review. Int J Pharm. 2011;415(1–2):34–52.
Middleton JC, Tipton AJ. Synthetic biodegradable polymers as orthopedic devices. Biomaterials. 2000;21(23):2335–46.
Chan S. Targeting the mammalian target of rapamycin (mTOR): a new approach to treating cancer. Br J Cancer. 2004;91(8):1420–4.
Schnadig I, Braun E, Mosier M, Geller RB, Lee S. Effect of APF530 on health-related quality of life (QOL) and other chemotherapy-induced nausea and vomiting (CINV) end points: phase III MAGIC trial [abstract]. J Oncol Pract: 2016 Am Soc Clin Oncol Annu Meet. 2016.
Wischke C, Schwendeman SP. Principles of encapsulating hydrophobic drugs in PLA/PLGA microparticles. Int J Pharm. 2008;364(2):298–327.
Zhao YM, Zhou Q, Xu Y, Lai XY, Huang H. Antiproliferative effect of rapamycin on human T-cell leukemia cell line Jurkat by cell cycle arrest and telomerase inhibition. Acta Pharmacol Sin. 2008;29(4):481–8.
Guba M, Koehl GE, Neppl E, Doenecke A, Steinbauer M, Schlitt HJ, et al. Dosing of rapamycin is critical to achieve an optimal antiangiogenic effect against cancer. Transpl Int. 2005;18(1):89–94.
Gallo R, Padurean A, Jayaraman T, Marx S, Roque M, Adelman S, et al. Inhibition of intimal thickening after balloon angioplasty in porcine coronary arteries by targeting regulators of the cell cycle. Circulation. 1999;99(16):2164–70.
Rivera VM, Ye X, Courage NL, Sachar J, Cerasoli Jr F, Wilson JM, et al. Long-term regulated expression of growth hormone in mice after intramuscular gene transfer. Proc Natl Acad Sci U S A. 1999;96(15):8657–62.
Kimball SR, Jefferson LS, Nguyen HV, Suryawan A, Bush JA, Davis TA. Feeding stimulates protein synthesis in muscle and liver of neonatal pigs through an mTOR-dependent process. Am J Physiol Endocrinol Metab. 2000;279(5):E1080–7.
Hackstein H, Taner T, Logar AJ, Thomson AW. Rapamycin inhibits macropinocytosis and mannose receptor-mediated endocytosis by bone marrow-derived dendritic cells. Blood. 2002;100(3):1084–7.
Acknowledgments
The authors would like to acknowledge the financial support from the Singapore Centre on Environmental Life Sciences Engineering (SCELSE) (M4330001.C70.703012), the School of Materials Science and Engineering (M020070110), the NTU-National Healthcare Group (NTU-NHG) grant (ARG/14012), Lee Kong Chian School of Medicine Postdoctoral Fellowship support for Han Wei Hou and the Lee Kong Chian School of Medicine, Nanyang Technological University start-up grant for Per-Olof Berggren.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fan, Y.L., Hou, H.W., Tay, H.M. et al. Preservation of Anticancer and Immunosuppressive Properties of Rapamycin Achieved Through Controlled Releasing Particles. AAPS PharmSciTech 18, 2648–2657 (2017). https://doi.org/10.1208/s12249-017-0745-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-017-0745-x