ABSTRACT
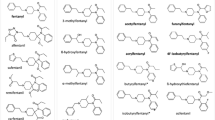
Carfentanil is an ultra-potent synthetic opioid. No human carfentanil metabolism data are available. Reportedly, Russian police forces used carfentanil and remifentanil to resolve a hostage situation in Moscow in 2002. This alleged use prompted interest in the pharmacology and toxicology of carfentanil in humans. Our study was conducted to identify human carfentanil metabolites and to assess carfentanil’s metabolic clearance, which could contribute to its acute toxicity in humans. We used Simulations Plus’s ADMET Predictor™ and Molecular Discovery’s MetaSite™ to predict possible metabolite formation. Both programs gave similar results that were generally good but did not capture all metabolites seen in vitro. We incubated carfentanil with human hepatocytes for up to 1 h and analyzed samples on a Sciex 3200 QTRAP mass spectrometer to measure parent compound depletion and extrapolated that to represent intrinsic clearance. Pooled primary human hepatocytes were then incubated with carfentanil up to 6 h and analyzed for metabolite identification on a Sciex 5600+ TripleTOF (QTOF) high-resolution mass spectrometer. MS and MS/MS analyses elucidated the structures of the most abundant metabolites. Twelve metabolites were identified in total. N-Dealkylation and monohydroxylation of the piperidine ring were the dominant metabolic pathways. Two N-oxide metabolites and one glucuronide metabolite were observed. Surprisingly, ester hydrolysis was not a major metabolic pathway for carfentanil. While the human liver microsomal system demonstrated rapid clearance by CYP enzymes, the hepatocyte incubations showed much slower clearance, possibly providing some insight into the long duration of carfentanil’s effects.





Similar content being viewed by others
References
Cole A, Mutlow A, Isaza R, et al. Pharmacokinetics and pharmacodynamics of carfentanil and naltrexone in female common eland (Taurotragus oryx). J Zoo Wildl Med. 2006;37(3):318–26.
Van Bever WF, Niemegeers CJ, Schellekens KH, Janssen PA. N-4-Substituted 1-(2-arylethyl)-4-piperidinyl-N-phenylpropanamides, a novel series of extremely potent analgesics with unusually high safety margin. Arzneimittelforschung. 1976;26(8):1548–51.
Subramanian G, Paterlini MG, Portoghese PS, Ferguson DM. Molecular docking reveals a novel binding site model for fentanyl at the mu-opioid receptor. J Med Chem. 2000;43(3):381–91.
Ling GS, Spiegel K, Nishimura SL, Pasternak GW. Dissociation of morphine’s analgesic and respiratory depressant actions. Eur J Pharmacol. 1983;86(3–4):487–8.
Shook JE, Watkins WD, Camporesi EM. Differential roles of opioid receptors in respiration, respiratory disease, and opiate-induced respiratory depression. Am Rev Respir Dis. 1990;142(4):895–909.
Newman A, Channing M, Finn R, et al. Ligands for imaging opioid receptors in conscious humans by positron emission tomography (PET). NIDA Res Monogr. 1988;90:117–21.
Endres CJ, Bencherif B, Hilton J, Madar I, Frost JJ. Quantification of brain mu-opioid receptors with [11C]carfentanil: reference-tissue methods. Nucl Med Biol. 2003;30(2):177–86.
Villemagne PS, Dannals RF, Ravert HT, Frost JJ. PET imaging of human cardiac opioid receptors. Eur J Nucl Med Mol Imaging. 2002;29(10):1385–8.
Krechetnkiov A. Moscow theatre siege: questions remain unanswered. British Broadcasting Corporation. 24 October 2012. Web. 13 May 2015.
Glasser SB, Baker P. Russia confirms suspicions about gas used in raid; Potent Anesthetic Pumped Into Theater; 2 More Hostages Die From Drug’s Effects. The Washington Post 31 October 2002: A15. Print.
Riches JR, Read RW, Black RM, Cooper NJ, Timperley CM. Analysis of clothing and urine from Moscow theatre siege casualties reveals carfentanil and remifentanil use. J Anal Toxicol. 2012;36(9):647–56.
Feierman DE, Lasker JM. Metabolism of fentanyl, a synthetic opioid analgesic, by human liver microsomes. Role of CYP3A4. Drug Metab Dispos. 1996;24(9):932–9.
Guitton J, Buronfosse T, Désage M, Lepape A, Brazier JL, Beaune P. Possible involvement of multiple cytochrome P450S in fentanyl and sufentanil metabolism as opposed to alfentanil. Biochem Pharmacol. 1997;53(11):1613–9.
Hanks GW, Hoskin PJ, Aherne GW, Turner P, Poulain P. Explanation for potency of repeated oral doses of morphine? Lancet. 1987;2(8561):723–5.
Schneider E, Brune K. Opioid activity and distribution of fentanyl metabolites. Naunyn-Schmiedeberg’s Arch Pharmacol. 1986;334(3):267–74.
ULTIVA® (remifentanil hydrochloride) [package insert]. Rockford, IL, USA; Mylan Institutional LLC; Published March 2015. http://www.mylan.com/en/products/product-catalog/product-profile-page?id=DA2E7ACD-ADCF-4DC1-A9F9-8ECBE954909B. Accessed 1 June 2015.
Bürkle H, Dunbar S, Van Aken H. Remifentanil: a novel, short-acting, mu-opioid. Anesth Analg. 1996;83(3):646–51.
Van Nimmen NF, Poels KL, Veulemans HA. Highly sensitive gas chromatographic-mass spectrometric screening method for the determination of picogram levels of fentanyl, sufentanil and alfentanil and their major metabolites in urine of opioid exposed workers. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;804(2):375–87.
Wohlfarth A, Castaneto MS, Zhu M, et al. Pentylindole/Pentylindazole synthetic cannabinoids and their 5-Fluoro analogs produce different primary metabolites: metabolite profiling for AB-PINACA and 5F-AB-PINACA. AAPS J. 2015;17(3):660–77.
Wang L, Bernert JT. Analysis of 13 fentanils, including sufentanil and carfentanil, in human urine by liquid chromatography-atmospheric-pressure ionization-tandem mass spectrometry. J Anal Toxicol. 2006;30(5):335–41.
Lavé T, Dupin S, Schmitt C, et al. The use of human hepatocytes to select compounds based on their expected hepatic extraction ratios in humans. Pharm Res. 1997;14(2):152–5.
Meuldermans WE, Hurkmans RM, Heykants JJ. Plasma protein binding and distribution of fentanyl, sufentanil, alfentanil and lofentanil in blood. Arch Int Pharmacodyn Ther. 1982;257(1):4–19.
Allen JL. Renarcotization following Carfentanil immobilization of nondomestic ungulates. J Zoo Wildl Med. 1989;20(4):423–6.
Thevis M, Geyer H, Bahr D, Schänzer W. Identification of fentanyl, alfentanil, sufentanil, remifentanil and their major metabolites in human urine by liquid chromatography/tandem mass spectrometry for doping control purposes. Eur J Mass Spectrom (Chichester, Eng). 2005;11(4):419–27.
Cashman JR, Park SB, Yang ZC, Wrighton SA, Jacob P, Benowitz NL. Metabolism of nicotine by human liver microsomes: stereoselective formation of trans-nicotine N’-oxide. Chem Res Toxicol. 1992;5(5):639–46.
Pirmohamed M, Williams D, Madden S, Templeton E, Park BK. Metabolism and bioactivation of clozapine by human liver in vitro. J Pharmacol Exp Ther. 1995;272(3):984–90.
Mcnaney CA, Drexler DM, Hnatyshyn SY, et al. An automated liquid chromatography-mass spectrometry process to determine metabolic stability half-life and intrinsic clearance of drug candidates by substrate depletion. Assay Drug Dev Technol. 2008;6(1):121–9.
Tetko IV, Gasteiger J, Todeschini R, Mauri A, Livingstone D, Ertl P, et al. Virtual computational chemistry laboratory - design and description. J Comput Aided Mol Des. 2005;19:453–63. article.
VCCLAB, Virtual Computational Chemistry Laboratory, http://www.vcclab.org; 2005.
Hansch C, Leo A. Exploring QSAR: hydrophobic, electronic, and steric constants. Vol. 2. ACS Publications. 1995.
ADMET Predictor v7.2.0001. Simulations Plus, Inc. 2015.
Hug CC, Murphy MR. Tissue redistribution of fentanyl and termination of its effects in rats. Anesthesiology. 1981;55(4):369–75.
Dart RC. Medical toxicology 3rd Edition. Phildelphia: Lippincott Williams and Wilkins; 2004. p. 762.
Henderson GL. Designer drugs: past history and future prospects. J Forensic Sci. 1988;33(2):569–75.
Mahlke NS, Ziesenitz V, Mikus G, Skopp G. Quantitative low-volume assay for simultaneous determination of fentanyl, norfentanyl, and minor metabolites in human plasma and urine by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Int J Legal Med. 2014;128(5):771–8.
Acknowledgments
The authors would like to acknowledge Drs. Alison Director-Myska, Eric Moore, Neil Jensen, and Joseph Corriveau for their unwavering and continued support of this research effort. We would also like to acknowledge Simulations Plus and Molecular Discovery for the use of their software for the in silico metabolite predictions. Finally, we would like to acknowledge Tim Moeller of BioreclamationIVT for his help with the primary hepatocyte culturing procedures and technical support.
This work was funded by the Defense Threat Reduction Agency (DTRA) under project number CB3281.
Author information
Authors and Affiliations
Corresponding author
Additional information
Michael G. Feasel and Ariane Wohlfarth contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 662 kb)
Rights and permissions
About this article
Cite this article
Feasel, M.G., Wohlfarth, A., Nilles, J.M. et al. Metabolism of Carfentanil, an Ultra-Potent Opioid, in Human Liver Microsomes and Human Hepatocytes by High-Resolution Mass Spectrometry. AAPS J 18, 1489–1499 (2016). https://doi.org/10.1208/s12248-016-9963-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-016-9963-5