Abstract
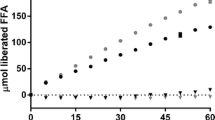
Solid self-microemulsifying drug delivery systems (SMEDDS) have received considerable attention in recent times attempting to overcome the drawbacks of liquid SMEDDS. Earlier literature reports on solid SMEDDS have focussed on formulation development; however, the digestibility and propensity for self-assembly of the digested components with endogenous bile salts and phospholipids are unknown. Therefore, as a starting point, previously reported solid SMEDDS containing Gelucire® 44/14 (GEL) and the non-digestible surfactants, Vitamin E TPGS (TPGS) and Lutrol® F 127 (F 127), were prepared, and their dispersion and digestion behaviours were studied using an in vitro lipolysis model, coupled with small-angle X-ray scattering (SAXS) to determine the formed colloidal structures during digestion in real time. GEL alone was digested (89%) and formed a lamellar phase (Lα). When surfactants were added at a 40:60% w/w lipid to surfactants ratio, digestion was inhibited with a significant lag time being evident. However, increasing the fraction of GEL to 50% w/w enabled digestion with reduced lag time. The substitution of the non-digestible surfactants with digestible surfactants, sucrose esters S-1670 (S-1670) and Span® 60 (S-60), eliminated the digestion lag time, and the formation of colloidal structures was more similar to that of GEL alone.








Similar content being viewed by others
REFERENCES
Vonderscher J, Meinzer A, editors. Rationale for the development of Sandimmune Neoral. Transplantation proceedings; 1994.
Pouton CW. Lipid formulations for oral administration of drugs: non-emulsifying, self-emulsifying and ’self-microemulsifying’ drug delivery systems. Eur J Pharm Sci. 2000;11(Supplement 2):S93–8.
Balakumar K, Raghavan CV, Selvan NT, Prasad RH, Abdu S. Self nanoemulsifying drug delivery system (SNEDDS) of rosuvastatin calcium: design, formulation, bioavailability and pharmacokinetic evaluation. Colloids Surf, B. 2013;112:337–43.
Gursoy RN, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother. 2004;58(3):173–82.
Čerpnjak K, Zvonar A, Gašperlin M, Vrečer F. Lipid-based systems as a promising approach for enhancing the bioavailability of poorly water-soluble drugs. Acta Pharm. 2013;63(4):427–45.
Truong DH, Tran TH, Ramasamy T, Choi JY, Lee HH, Moon C, et al. Development of solid self-emulsifying formulation for improving the oral bioavailability of Erlotinib. AAPS PharmSciTech. 2015:1-8.
Cerpnjak K, Zvonar A, Vrecer F, Gasperlin M. Development of a solid self-microemulsifying drug delivery system (SMEDDS) for solubility enhancement of naproxen. Drug Dev Ind Pharm. 2015;41(9):1548–57.
Pouton CW, Porter CJH. Formulation of lipid-based delivery systems for oral administration: materials, methods and strategies. Adv Drug Delivery Rev. 2008;60(6):625–37.
Strickley RG. Solubilizing excipients in oral and injectable formulations. Pharm Res. 2004;21(2):201–30.
Nazzal S, Wang Y. Characterization of soft gelatin capsules by thermal analysis. Int J Pharm. 2001;230(1–2):35–45.
Mei X, Etzler FM, Wang Z. Use of texture analysis to study hydrophilic solvent effects on the mechanical properties of hard gelatin capsules. Int J Pharm. 2006;324(2):128–35.
Kalepu S, Manthina M, Padavala V. Oral lipid-based drug delivery systems—an overview. Acta Pharmaceutica Sinica B. 2013;3(6):361–72.
Boltri L, Coceani N, Curto DD, Dobetti L, Esposito P. Enhancement and modification of etoposide release from crospovidone particles loaded with oil-surfactant blends. Pharm Dev Technol. 1997;2(4):373–81.
Oh DH, Kang JH, Kim DW, Lee B-J, Kim JO, Yong CS, et al. Comparison of solid self-microemulsifying drug delivery system (solid SMEDDS) prepared with hydrophilic and hydrophobic solid carrier. Int J Pharm. 2011;420(2):412–8.
Venkatesan N, Yoshimitsu J, Ito Y, Shibata N, Takada K. Liquid filled nanoparticles as a drug delivery tool for protein therapeutics. Biomaterials. 2005;26(34):7154–63.
Pouton CW. Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. Eur J Pharm Sci. 2006;29(3–4):278–87.
Passerini N, Albertini B, Perissutti B, Rodriguez L. Evaluation of melt granulation and ultrasonic spray congealing as techniques to enhance the dissolution of praziquantel. Int J Pharm. 2006;318(1):92–102.
Rodriguez L, Passerini N, Cavallari C, Cini M, Sancin P, Fini A. Description and preliminary evaluation of a new ultrasonic atomizer for spray-congealing processes. Int J Pharm. 1999;183(2):133–43.
Yi T, Wan J, Xu H, Yang X. A new solid self-microemulsifying formulation prepared by spray-drying to improve the oral bioavailability of poorly water soluble drugs. Eur J Pharm Biopharm. 2008;70(2):439–44.
Singh SK, Vuddanda PR, Singh S, Srivastava AK. A comparison between use of spray and freeze drying techniques for preparation of solid self-microemulsifying formulation of valsartan and in vitro and in vivo evaluation. BioMed research international. 2013;2013.
Nanda Kishore R, Yalavarthi PR, Vadlamudi HC, Vandana K, Rasheed A, Sushma M. Solid self microemulsification of Atorvastatin using hydrophilic carriers: a design. Drug Dev Ind Pharm. 2015;41(7):1213–22.
Seo A, Holm P, Kristensen HG, Schæfer T. The preparation of agglomerates containing solid dispersions of diazepam by melt agglomeration in a high shear mixer. Int J Pharm. 2003;259(1–2):161–71.
Gupta M, Tseng Y-C, Goldman D, Bogner R. Hydrogen bonding with adsorbent during storage governs drug dissolution from solid-dispersion granules. Pharm Res. 2002;19(11):1663–72.
Gupta MK, Goldman D, Bogner RH, Tseng Y-C. Enhanced drug dissolution and bulk properties of solid dispersions granulated with a surface adsorbent. Pharm Dev Technol. 2001;6(4):563–72.
Sethia S, Squillante E. Physicochemical characterization of solid dispersions of carbamazepine formulated by supercritical carbon dioxide and conventional solvent evaporation method. J Pharm Sci. 2002;91(9):1948–57.
Thies C, Dos Santos IR, Richard J, VandeVelde V, Rolland H, Benoit J-P. A supercritical fluid-based coating technology 1: process considerations. J Microencapsulation. 2003;20(1):87–96.
Sethia S, Squillante E. Solid dispersion of carbamazepine in PVP K30 by conventional solvent evaporation and supercritical methods. Int J Pharm. 2004;272(1):1–10.
P. Thipsay JM, M. Repka hot melt extrusion technology for the formulation of solid self microemulsifying drug delivery systems. AAPS 2014.
Kossena GA, Charman WN, Boyd BJ, Porter CJH. Influence of the intermediate digestion phases of common formulation lipids on the absorption of a poorly water-soluble drug. J Pharm Sci. 2005;94(3):481–92.
Porter CJ, Kaukonen AM, Taillardat‐Bertschinger A, Boyd BJ, O’Connor JM, Edwards GA, et al. Use of in vitro lipid digestion data to explain the in vivo performance of triglyceride‐based oral lipid formulations of poorly water‐soluble drugs: studies with halofantrine. J Pharm Sci. 2004;93(5):1110–21.
Porter CJ, Trevaskis NL, Charman WN. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discovery. 2007;6(3):231–48.
Kossena GA, Boyd BJ, Porter CJ, Charman WN. Separation and characterization of the colloidal phases produced on digestion of common formulation lipids and assessment of their impact on the apparent solubility of selected poorly water‐soluble drugs. J Pharm Sci. 2003;92(3):634–48.
MacGregor KJ, Embleton JK, Lacy JE, Perry EA, Solomon LJ, Seager H, et al. Influence of lipolysis on drug absorption from the gastro-intestinal tract. Adv Drug Delivery Rev. 1997;25(1):33–46.
Zangenberg NH, Müllertz A, Kristensen HG, Hovgaard L. A dynamic in vitro lipolysis model: I. controlling the rate of lipolysis by continuous addition of calcium. Eur J Pharm Sci. 2001;14(2):115–22.
Fernandez S, Rodier J-D, Ritter N, Mahler B, Demarne F, Carrière F, et al. Lipolysis of the semi-solid self-emulsifying excipient Gelucire® 44/14 by digestive lipases. Biochim Biophys Acta (BBA)-Mol Cell Biol Lipids. 2008;1781(8):367–75.
Warren DB, Anby MU, Hawley A, Boyd BJ. Real time evolution of liquid crystalline nanostructure during the digestion of formulation lipids using synchrotron small-angle X-ray scattering. Langmuir. 2011;27(15):9528–34.
Zhang Z, Tan S, Feng S-S. Vitamin E TPGS as a molecular biomaterial for drug delivery. Biomaterials. 2012;33(19):4889–906.
Kabanov AV, Batrakova EV, Alakhov VY. Pluronic® block copolymers as novel polymer therapeutics for drug and gene delivery. J Controlled Release. 2002;82(2):189–212.
Mitsubishi Kagaku Foods Corporation J. http://www.mfccojp/english/s1670.htm
National Library of Medicine TdN. https://www.toxnetnlmnihgov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+778
Craig D, Barker S, Banning D, Booth S. An investigation into the mechanisms of self-emulsification using particle size analysis and low frequency dielectric spectroscopy. Int J Pharm. 1995;114(1):103–10.
Trull A, Tan K, Tan L, Alexander G, Jamieson N, editors. Enhanced absorption of new oral cyclosporin microemulsion formulation, Neoral, in liver transplant recipients with external biliary diversion. Transplantation proceedings; 1994.
Chamieh J, Davanier F, Jannin V, Demarne F, Cottet H. Size characterization of commercial micelles and microemulsions by Taylor dispersion analysis. Int J Pharm. 2015;492(1–2):46–54.
Khan J, Hawley A, Rades T, Boyd BJ. In situ lipolysis and synchrotron small-angle X-ray scattering for the direct determination of the precipitation and solid-state form of a poorly water-soluble drug during digestion of a lipid-based formulation. Journal of Pharmaceutical Sciences.
Phan S, Salentinig S, Prestidge CA, Boyd BJ. Self-assembled structures formed during lipid digestion: characterization and implications for oral lipid-based drug delivery systems. Drug Deliv Transl Res. 2014;4(3):275–94.
Kaukonen AM, Boyd BJ, Charman WN, Porter CJ. Drug solubilization behavior during in vitro digestion of suspension formulations of poorly water-soluble drugs in triglyceride lipids. Pharm Res. 2004;21(2):254–60.
Sek L, Porter CJ, Kaukonen AM, Charman WN. Evaluation of the in‐vitro digestion profiles of long and medium chain glycerides and the phase behaviour of their lipolytic products. J Pharm Pharmacol. 2002;54(1):29–41.
Gargouri Y, Moreau H, Verger R. Gastric lipases: biochemical and physiological studies. Biochim Biophys Acta. 1989;1006(3):255.
Dressman JB, Berardi RR, Dermentzoglou LC, Russell TL, Schmaltz SP, Barnett JL, et al. Upper gastrointestinal (GI) pH in young, healthy men and women. Pharm Res. 1990;7(7):756–61.
Mu L, Feng S. A novel controlled release formulation for the anticancer drug paclitaxel (Taxol®): PLGA nanoparticles containing vitamin E TPGS. J Controlled Release. 2003;86(1):33–48.
Vitamin E TPGS, properties and applications. Eastman, USA.Publication EFC-226A(October 1998).
Szűts A. Study of the applicability of sucrose esters in hot-melt technology. PhD Faculty of Pharmacy, Department of Pharmaceutical Technology University of Szeged. 2003.
Christiansen A, Backensfeld T, Weitschies W. Effects of non-ionic surfactants on in vitro triglyceride digestion and their susceptibility to digestion by pancreatic enzymes. Eur J Pharm Sci. 2010;41(2):376–82.
Sagitani H. Making homogeneous and fine droplet O/W emulsions using nonionic surfactants. J Am Oil Chem Soc. 1981;58(6):738–43.
Gullapalli RP, Sheth BB. Influence of an optimized non-ionic emulsifier blend on properties of oil-in-water emulsions. Eur J Pharm Biopharm. 1999;48(3):233–8.
Jannin V, Pochard E, Chambin O. Influence of poloxamers on the dissolution performance and stability of controlled-release formulations containing Precirol® ATO 5. Int J Pharm. 2006;309(1):6–15.
Goddeeris C, Van den Mooter G. Free flowing solid dispersions of the anti-HIV drug UC 781 with Poloxamer 407 and a maximum amount of TPGS 1000: investigating the relationship between physicochemical characteristics and dissolution behaviour. Eur J Pharm Sci. 2008;35(1–2):104–13.
Guo Y, Luo J, Tan S, Otieno BO, Zhang Z. The applications of vitamin E TPGS in drug delivery. Eur J Pharm Sci. 2013;49(2):175–86.
Shah DO. Macro- and microemulsions: theory and applications: ACS Publications.
Wulff-Pérez M, de Vicente J, Martín-Rodríguez A, Gálvez-Ruiz MJ. Controlling lipolysis through steric surfactants: new insights on the controlled degradation of submicron emulsions after oral and intravenous administration. Int J Pharm. 2012;423(2):161–6.
Feeney OM, Williams HD, Pouton CW, Porter CJ. ‘Stealth’lipid-based formulations: poly (ethylene glycol)-mediated digestion inhibition improves oral bioavailability of a model poorly water soluble drug. J Controlled Release. 2014;192:219–27.
ACKNOWLEDGEMENTS
Kapilkumar’s Ph.D. research was supported by Gattefossé (Saint-Priest, France). The SAXS experiments were undertaken at the SAXS/WAXS beamline at the Australian Synchrotron, Victoria, Australia. Ben Boyd is funded under the Australian Research Council Future Fellowship scheme, and the project was partly funded by the ARC Discovery Grant scheme.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
Vincent Jannin is an employee of Gattefossé (Saint-Priest, France), who manufactures the Gelucire® 44/14 used in this study.
Rights and permissions
About this article
Cite this article
Vithani, K., Hawley, A., Jannin, V. et al. Inclusion of Digestible Surfactants in Solid SMEDDS Formulation Removes Lag Time and Influences the Formation of Structured Particles During Digestion. AAPS J 19, 754–764 (2017). https://doi.org/10.1208/s12248-016-0036-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-016-0036-6