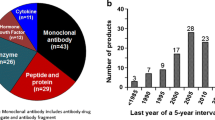
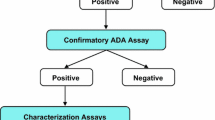
Abstract
Immunogenicity is a significant concern for biologic drugs as it can affect both safety and efficacy. To date, the descriptions of product immunogenicity have varied not only due to different degrees of understanding of product immunogenicity at the time of licensing but also due to an evolving lexicon that has generated some confusion in the field. In recent years, there has been growing consensus regarding the data needed to assess product immunogenicity. Harmonization of the strategy for the elucidation of product immunogenicity by drug developers, as well as the use of defined common terminology, can benefit medical practitioners, health regulatory agencies, and ultimately the patients. Clearly, understanding the incidence, kinetics and magnitude of anti-drug antibody (ADA), its neutralizing ability, cross-reactivity with endogenous molecules or other marketed biologic drugs, and related clinical impact may enhance clinical management of patients treated with biologic drugs. To that end, the authors present terms and definitions for describing and analyzing clinical immunogenicity data and suggest approaches to data presentation, emphasizing associations of ADA development with pharmacokinetics, efficacy, and safety that are necessary to assess the clinical relevance of immunogenicity.






Similar content being viewed by others
References
Schellekens H. Bioequivalence and the immunogenicity of biopharmaceuticals. Nat Rev Drug Discov. 2002;1(6):457–62. doi:10.1038/nrd818.
Schnabel CA, Fineberg SE, Kim DD. Immunogenicity of xenopeptide hormone therapies. Peptides. 2006;27(7):1902–10. doi:10.1016/j.peptides.2006.01.019.
Kuus-Reichel K, Grauer LS, Karavodin LM, Knott C, Krusemeier M, Kay NE. Will immunogenicity limit the use, efficacy, and future development of therapeutic monoclonal antibodies? Clin Diagn Lab Immunol. 1994;1(4):365–72.
Koren E, Zuckerman LA, Mire-Sluis AR. Immune responses to therapeutic proteins in humans—clinical significance, assessment and prediction. Curr Pharm Biotechnol. 2002;3(4):349–60.
Schellekens H, Casadevall N. Immunogenicity of recombinant human proteins: causes and consequences. J Neurol. 2004;251 Suppl 2:II4–9. doi:10.1007/s00415-004-1202-9.
Mayer L, Young Y. Infusion reactions and their management. Gastroenterol Clin N Am. 2006;35(4):857–66. doi:10.1016/j.gtc.2006.09.006.
Scheinfeld N. A comprehensive review and evaluation of the side effects of the tumor necrosis factor alpha blockers etanercept, infliximab and adalimumab. J Dermatol Treat. 2004;15(5):280–94. doi:10.1080/09546630410017275.
D’Arcy CA, Mannik M. Serum sickness secondary to treatment with the murine-human chimeric antibody IDEC-C2B8 (rituximab). Arthritis Rheum. 2001;44(7):1717–8. doi:10.1002/1529-0131(200107)44:7<1717::AID-ART299>3.0.CO;2-C.
Korswagen LA, Bartelds GM, Krieckaert CL, Turkstra F, Nurmohamed MT, van Schaardenburg D, et al. Venous and arterial thromboembolic events in adalimumab-treated patients with antiadalimumab antibodies: a case series and cohort study. Arthritis Rheum. 2011;63(4):877–83. doi:10.1002/art.30209.
Bartelds GM, Wijbrandts CA, Nurmohamed MT, Stapel S, Lems WF, Aarden L, et al. Clinical response to adalimumab: relationship to anti-adalimumab antibodies and serum adalimumab concentrations in rheumatoid arthritis. Ann Rheum Dis. 2007;66(7):921–6. doi:10.1136/ard.2006.065615.
Wolbink GJ, Aarden LA, Dijkmans BA. Dealing with immunogenicity of biologicals: assessment and clinical relevance. Curr Opin Rheumatol. 2009;21(3):211–5.
Yanai H, Hanauer SB. Assessing response and loss of response to biological therapies in IBD. Am J Gastroenterol. 2011;106(4):685–98. doi:10.1038/ajg.2011.103.
Casadevall N, Nataf J, Viron B, Kolta A, Kiladjian JJ, Martin-Dupont P, et al. Pure red-cell aplasia and antierythropoietin antibodies in patients treated with recombinant erythropoietin. N Engl J Med. 2002;346(7):469–75. doi:10.1056/NEJMoa011931.
Macdougall IC. Antibody-mediated pure red cell aplasia (PRCA): epidemiology, immunogenicity and risks. Nephrol Dial Transplant. 2005;20 Suppl 4:iv9–iv15.
Guideline On Immunogenicity Assessment Of Biotechnology-Derived Therapeutic Proteins, EMEA/CHMP/BMWP/14327/2006. http://www.tga.gov.au/pdf/euguide/bmwp1432706en.pdf. Accessed 18 Mar 2014.
Janeway C. Immunogenicity signals 1,2,3 … and 0. Immunol Today. 1989;10(9):283–6.
Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13(1):114–9.
Schellekens H. Immunogenicity of therapeutic proteins: clinical implications and future prospects. Clin Ther. 2002;24(11):1720–40. discussion 19.
Shankar G, Pendley C, Stein KE. A risk-based bioanalytical strategy for the assessment of antibody immune responses against biological drugs. Nat Biotechnol. 2007;25(5):555–61. doi:10.1038/nbt1303.
Koren E, Smith HW, Shores E, Shankar G, Finco-Kent D, Rup B, et al. Recommendations on risk-based strategies for detection and characterization of antibodies against biotechnology products. J Immunol Methods. 2008;333(1–2):1–9. doi:10.1016/j.jim.2008.01.001.
Ponce R, Abad L, Amaravadi L, Gelzleichter T, Gore E, Green J, et al. Immunogenicity of biologically-derived therapeutics: assessment and interpretation of nonclinical safety studies. Regul Toxicol Pharmacol. 2009;54(2):164–82. doi:10.1016/j.yrtph.2009.03.012.
Jahn EM, Schneider CK. How to systematically evaluate immunogenicity of therapeutic proteins—regulatory considerations. New Biotechnol. 2009;25(5):280–6. doi:10.1016/j.nbt.2009.03.012.
Guidance for Industry: Assay Development for Immunogenicity Testing of Therapeutic Proteins. U.S. Department of Health and Human Services (DHHS), Food and Drug Administration (FDA), Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). http://www.fda.gov/downloads/Drugs/…/Guidances/UCM192750.pdf. Accessed 18 Mar 2014.
Shankar G, Devanarayan V, Amaravadi L, Barrett YC, Bowsher R, Finco-Kent D, et al. Recommendations for the validation of immunoassays used for detection of host antibodies against biotechnology products. J Pharm Biomed Anal. 2008;48(5):1267–81. doi:10.1016/j.jpba.2008.09.020.
Buttel IC, Chamberlain P, Chowers Y, Ehmann F, Greinacher A, Jefferis R, et al. Taking immunogenicity assessment of therapeutic proteins to the next level. Biologicals. 2011;39(2):100–9. doi:10.1016/j.biologicals.2011.01.006.
Wang YM, Fang L, Zhou L, Wang J, Ahn HY. A survey of applications of biological products for drug interference of immunogenicity assays. Pharm Res. 2012;29(12):3384–92. doi:10.1007/s11095-012-0833-2.
Guidance for industry: immunogenicity assessment for therapeutic protein products. In: U.S. Department of Health and Human Services (DHHS), Food and Drug Administration (FDA), Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM338856.pdf2013. Accessed 18 Mar 2014.
Naserke HE, Bonifacio E, Ziegler AG. Prevalence, characteristics and diabetes risk associated with transient maternally acquired islet antibodies and persistent islet antibodies in offspring of parents with type 1 diabetes. J Clin Endocrinol Metab. 2001;86(10):4826–33. doi:10.1210/jcem.86.10.7931.
Male C, Foulon D, Hoogendoorn H, Vegh P, Silverman E, David M, et al. Predictive value of persistent versus transient antiphospholipid antibody subtypes for the risk of thrombotic events in pediatric patients with systemic lupus erythematosus. Blood. 2005;106(13):4152–8. doi:10.1182/blood-2005-05-2048.
Calabresi PA, Giovannoni G, Confavreux C, Galetta SL, Havrdova E, Hutchinson M, et al. The incidence and significance of anti-natalizumab antibodies: results from AFFIRM and SENTINEL. Neurology. 2007;69(14):1391–403. doi:10.1212/01.wnl.0000277457.17420.b5.
Chirmule N, Jawa V, Meibohm B. Immunogenicity to therapeutic proteins: impact on PK/PD and efficacy. AAPS J. 2012;14(2):296–302. doi:10.1208/s12248-012-9340-y.
Guidance for industry: immunotoxicology evaluation of investigational new drugs. U.S. Department of Health and Human Services (DHHS), Food and Drug Administration (FDA), Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm079239.pdf2002. Accessed 18 Mar 2014.
Bartelds GM, Krieckaert CL, Nurmohamed MT, van Schouwenburg PA, Lems WF, Twisk JW, et al. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA. 2011;305(14):1460–8. doi:10.1001/jama.2011.406.
Ducourau E, Mulleman D, Paintaud G, Miow Lin DC, Lauferon F, Ternant D, et al. Antibodies toward infliximab are associated with low infliximab concentration at treatment initiation and poor infliximab maintenance in rheumatic diseases. Arthritis Res Ther. 2011;13(3):R105. doi:10.1186/ar3386.
Beck JR, Shultz EK. The use of relative operating characteristic (ROC) curves in test performance evaluation. Arch Pathol Lab Med. 1986;110(1):13–20.
Steenholdt C, Bendtzen K, Brynskov J, Thomsen OO, Ainsworth MA. Cut-off levels and diagnostic accuracy of infliximab trough levels and anti-infliximab antibodies in Crohn’s disease. Scand J Gastroenterol. 2011;46(3):310–8. doi:10.3109/00365521.2010.536254.
Rosenberg AS, Worobec A. A risk-based approach to immunogenicity concerns of therapeutic protein products—Part 1–considering consequences of the immune response to a protein. Biopharm Int. 2004;17:22–6.
Rosenberg AS, Worobec A. A risk-based approach to immunogenicity concerns of therapeutic protein products—Part 2–considering host-specific and product-specific factors impacting immunogenicity. Biopharm Int. 2004;17:34–42.
Mire-Sluis AR, Barrett YC, Devanarayan V, Koren E, Liu H, Maia M, et al. Recommendations for the design and optimization of immunoassays used in the detection of host antibodies against biotechnology products. J Immunol Methods. 2004;289(1–2):1–16. doi:10.1016/j.jim.2004.06.002.
Gupta S, Indelicato SR, Jethwa V, Kawabata T, Kelley M, Mire-Sluis AR, et al. Recommendations for the design, optimization, and qualification of cell-based assays used for the detection of neutralizing antibody responses elicited to biological therapeutics. J Immunol Methods. 2007;321(1–2):1–18. doi:10.1016/j.jim.2006.12.004.
Gupta S, Devanarayan V, Finco D, Gunn 3rd GR, Kirshner S, Richards S, et al. Recommendations for the validation of cell-based assays used for the detection of neutralizing antibody immune responses elicited against biological therapeutics. J Pharm Biomed Anal. 2011;55(5):878–88. doi:10.1016/j.jpba.2011.03.038.
Rosenberg AS, Worobec A. A risk-based approach to immunogenicity concerns of therapeutic protein products—Part 3–effects of manufacturing changes in immunogenicity and the utility of animal immunogenicity studies. Biopharm Int. 2005;18:32–6.
Guideline on immunogenicity assessment of monoclonal antibodies intended for in vivo clinical use. EMA/CHMP/BMWP/86289/2010. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500128688.pdf. Accessed 18 Mar 2014.
Lofgren JA, Dhandapani S, Pennucci JJ, Abbott CM, Mytych DT, Kaliyaperumal A, et al. Comparing ELISA and surface plasmon resonance for assessing clinical immunogenicity of panitumumab. J Immunol. 2007;178(11):7467–72.
Li J, Schantz A, Schwegler M, Shankar G. Detection of low-affinity anti-drug antibodies and improved drug tolerance in immunogenicity testing by Octet((R)) biolayer interferometry. J Pharm Biomed Anal. 2011;54(2):286–94. doi:10.1016/j.jpba.2010.08.022.
Dai S, Schantz A, Clements-Egan A, Cannon M, Shankar G. Development of a method that eliminates false positive results due to nerve growth factor interference in the assessment of fulranumab immunogenicity. AAPS J. 2014; doi:10.1208/s12248-014-9581-z.
Kelley M, Ahene AB, Gorovits B, Kamerud J, King LE, McIntosh T, et al. Theoretical considerations and practical approaches to address the effect of anti-drug antibody (ADA) on quantification of biotherapeutics in circulation. AAPS J. 2013;15(3):646–58. doi:10.1208/s12248-013-9468-4.
Acknowledgments
This work was sponsored by The Therapeutic Protein Immunogenicity Focus Group (TPIFG) of the BIOTEC Section, American Association of Pharmaceutical Scientists (AAPS). A global physician survey was conducted by TPIFG in 2010 and 2011 to assess the viewpoints and needs of medical practitioners relative to immunogenicity. Survey results and a call for the harmonization of terminology and the analysis and reporting of clinical immunogenicity were presented at the European Immunogenicity Platform (EIP) Symposium in December 2010 (Gent, Belgium) and at an Open Forum of the AAPS National Biotechnology Conference (NBC) in May 2011 (San Francisco, USA). Draft definitions and work-in-progress data presentation tactics were presented at the EIP symposia in February 2012 (Copenhagen, Denmark) and February 2013 (Munich, Germany) and at AAPS-NBC conventions in May 2012 and May 2013 (San Diego, USA). Finally, a draft manuscript was posted on the AAPS website for public feedback. The authors thank all those who provided feedback, which was considered during the finalization of this manuscript. During the preparation of this manuscript, our recommended terminology, definitions, and approaches for ADA characterization were shared with the ABIRISK (Anti-Biopharmaceutical Immunization: prediction and analysis of clinical relevance to minimize the RISK) consortium, which agreed to adopt them. ABIRISK is a project of the Innovative Medicines Initiative (IMI), a public-private partnership between the European Union and the European Federation of Pharmaceutical Industries and Associations (EFPIA), and aims to conduct immunogenicity studies of several biologic drugs, which will be used to develop a database comprising their evaluations of factors underlying immunogenicity and to generate tools for determining how individual patients are likely to respond.
Disclaimer
The contents of this article reflect the personal opinions of the authors and may not represent the official positions or perspectives of their affiliated organizations.
Author information
Authors and Affiliations
Corresponding author
Additional information
The contents of this article reflect the consensus perspective of the authors and may not represent the official positions or expectations of their affiliated organizations. This article is not a substitute or equivalent of a guidance document from a health regulatory agency.
Rights and permissions
About this article
Cite this article
Shankar, G., Arkin, S., Cocea, L. et al. Assessment and Reporting of the Clinical Immunogenicity of Therapeutic Proteins and Peptides—Harmonized Terminology and Tactical Recommendations. AAPS J 16, 658–673 (2014). https://doi.org/10.1208/s12248-014-9599-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-014-9599-2