Abstract
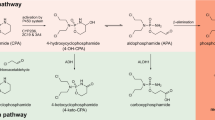
It has been reported that the toxicity of carmustine (BCNU) cyclophosphamide (CY)/etoposide regimen (when BCNU is split into 4 doses) is less than that of BCNU/CY/cisplatin regimen (when the same amount of BCNU is administered as a single dose). We hypothesized that this might in part be due to the inhibition of aldehyde dehydrogenase 1 (ALDH1) by BCNU or its degradation product, 2-chloroethyl isocyanate, which is likely to be more pronounced at the higher BCNU dose. The effects of BCNU and 2-chloroethyl isocyanate on the formation of carboxyethylphosphoramide mustard (CEPM) from 4-hydroxycyclophosphamide (HCY) was evaluated in human liver cytosol incubations. We found that CEPM formation from HCY was inhibited strongly by BCNU and weakly by 2-chloroethyl isocyanate. The mechanism of inhibition of ALDH1 activity by BCNU was elucidated using indole-3-acetaldehyde (IAL) as the probe substrate in ALDH1 prepared from human erythrocytes. BCNU was a competitive inhibitor of ALDH1 activity with a Ki of 1.95 μM. The inhibition was independent of preincubation time and reversible by dialysis. The calculated %inhibition of ALDH1 activity by acrolein and BCNU in patients receiving BCNU in 4 split doses with CY was 81%, and it increased to 92% in single dose BCNU regimen. Thus, the calculation indicates that residual operating ALDH1 activity is halved in the presence of single-dose BCNU compared to split-dose BCNU. The inhibition of ALDH1 may contribute to the observed lower incidence of toxicity when BCNU was split into 4 doses compared with single dose and coadministered with CY although dose-dependent effects of BCNU on glutathione and glutathione reductase are also likely to contribute.
Similar content being viewed by others
Abbreviations
- CY:
-
cyclophosphamide
- BCNU:
-
carmustine
- HCY:
-
4-hydroxycyclophosphamide
- CEPM:
-
carboxyethylphosphoramide mustard
- ALDH1:
-
aldehyde dehydrogenase 1
References
Ren, S., Yang, J.-S., Kalhorn, T. F., and Slattery, J. T. Oxidation of cyclophosphamide to 4-hydroxycyclophosphamide and deschloroethylcyclophosphamide in human liver microsomes. Cancer Res. 57, 4229–4235, 1997.
Chang, T. K. H., Yu, L., Goldstein, J. A., and Waxman, D. J. Identification of the polymorphically expressed CYP21 and the wild-type CYP2C9-ILE359 allele as low-K-m catalysts of cyclophosphamide and ifosfamide activation. Pharmacogenetics, 7: 211–221, 1997.
Dockham, P. A., Lee, M.-O., and Sladek, N. E. Identification of human liver aldehyde dehydrogenases that catalyze the oxidation of aldophosphamide and retinaldehyde. Biochem Pharmacol., 43: 2453–2469, 1992.
Parekh, H. K. and Sladek, N. E. NADPH-dependent enzymecatalyzed reduction of aldophosphamide, the pivotal metabolite of cyclophosphamide. Biochem. Pharmacol., 46: 1043–1052, 1993.
Sladek, N. E. Metabolism and pharmacokinetics of cyclophosphamide and related oxazaphosphorines. In: G. Powis (ed.) Anticancer Drugs: Reactive Metabolism and Drug Interactions, pp. 79–156, New York: Pergamon Press Ltd., 1994.
Devita, V. T., Hellman, S., and Rosenberg, S. A. Cancer Principles and Practice of Oncology. Philadelphia: Lippincott, 1985.
Colvin, M. and Brundrett, R. Chemical decomposition of chloroethylnitrosoureas. In: A. W. Prestakyo, S. T. Crooke, S. K. Baker, and P. S. Schein (eds.), Nitrosoureas, pp. 43–49. New York. Academic Press, 1981.
Ludlum, D. B. and Tong, W. P. Modification of DNA and RNA bases. In: A. W. Prestakyo, S. T. Crooke, S. K. Baker, and P. S. Schein (eds.), Nitrosoureas, pp. 85–94. New York: Academic Press, 1981.
Ayash, L. J., Hunt, M., Antman, K., Nadler, L., Wheeler, C., Takvorian, T., Elias, A., Antin, J. H., Greenough, T., and Eder, J. P. Hepatic venoocclusive disease in autologous bone marrow transplantation of solid tumors and lymphomas. J. Clin. Oncol., 8: 1699–1706, 1990.
Nagasawa, H. T., Elberling, J. A., Good, D. J., and Shirota, F. N. Latent alkyl isocyanates as inhibitors of aldehyde dehydrogenase in vivo. J Med. Chem., 37: 4222–4226, 1994.
Takamizawa, A., Matsumoto, S., Iwata, T., Tochino, Y., Katagiri, K., and Yamaguchi, K. Studies on cyclophosphamide metabolites and their related compounds. II. Preparation of an active species of cyclophosphamide and related compounds. J. Med. Chem., 18: 376–83, 1975.
Bradford, M. A. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of proteindye binding. Anal. Biochem., 72: 248–254, 1976.
Yoshida, A., Dave, V., Ward, R. J., and Peters, T. J. Cytosolic aldehyde dehydrogenase (ALDH1) variants found in alcohol flushers. Ann Hum Genet., 53: 1–7, 1989.
Ren, S., Kalhorn, T. F., McDonald, G. B., Anasetti, C., Appelbaum, F. R., and Slattery, J. T. Pharmacokinetics of Cyclophosphamide and its Metabolites in Bone Marrow Transplantation Patients. Clin Pharmacol. Ther., 64: 289–301, 1998.
Segel, I. H. Enzyme Kinetics. New York John Wiley & Sons. 1975.
Ren, S., Kalhorn, T. F., and Slattery, J. T. Inhibition of human aldehyde dehydrogenase 1 by the 4-hydroxycyclophosphamide degradation product acrolein Drug. Met. Dispos. In press
DeLeve, L. D. Cellular target of cyclophosphamide toxicity in the murine liver: Role of glutathione and site of metabolic activation. Hepatology, 24: 830–837, 1996.
Alarcon, R. A. Studies on the in vivo formation of acrolein: 3-hydroxypropylmercapturic acid as an index of cyclophosphamide (NSC-26271) activation. Cancer Treat. Rep., 60: 327–335, 1976.
Frischer, H. and Ahmad, T. Consequences of erythrocytic glutathione reductase deficiency. J Lab Clin. Med., 109: 583–588. 1987.
Frischer, H., Kennedy, E. J., Chigurupati, R., and Sivarajan, M., Glutathrone, cell proliferation, and 1,3-bis-(2-chloroethyl)-1-nitrosourea in K562 leukemia. J Clin Invest, 92: 2761–2767, 1993.
Chang, T. K. H., Yu, L., Maurel, P., and Waxman, D. J. Enhanced cyclophosphamide and ifosfamide activation in primary human hepatocyte cultures: Response to cytochrome P.-450 inducers and autoinduction by oxazaphosphorines. Cancer Res, 57: 1946–1954, 1997.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published: September 20, 1999.
Rights and permissions
About this article
Cite this article
Ren, S., Slattery, J.T. Inhibition of carboxyethylphosphoramide mustard formation from 4-hydroxycyclophosphamide by carmustine. AAPS PharmSci 1, 14 (1999). https://doi.org/10.1208/ps010314
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/ps010314