Abstract
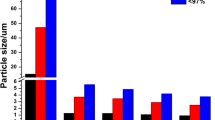
For lung transplant patients, a respirable, inulin-based solid dispersion containing cyclosporine A (CsA) has been developed. The solid dispersions were prepared by spray freezedrying. The solid dispersion was characterized by water vapor uptake, specific surface area analysis, and particle size analysis. Furthermore, the mode of inclusion of CsA in the dispersion was investigated with Fourier transform infrared spectroscopy. Finally, the dissolution behavior was determined and the aerosol that was formed by the powder was characterized. The powder had large specific surface areas (∼160 m2). The water vapor uptake was dependent linearly on the drug load. The type of solid dispersion was a combination of a solid solution and solid suspension. At a 10% drug load, 55% of the CsA in the powder was in the form of a solid solution and 45% as solid suspension. At 50% drug load, the powder contained 90% of CsA as solid suspension. The powder showed excellent dispersion characteristics as shown by the high emitted fraction (95%), respirable fraction (75%), and fine-particle fraction (50%). The solid dispersions consisted of relatively large (x50≈7 μm), but low-density particles (ρ≈0.2 g/cm3). The solid dispersions dissolved faster than the physical mixture, and inulin dissolved faster than CsA. The spray freeze-drying with inulin increased the specific surface area and wettability of CsA. In conclusion, the developed powder seems suitable for inhalation in the local treatment of lung transplant patients.
Similar content being viewed by others
References
Corcoran TE, Smaldone GC, Dauber JH, et al. Preservation of posttransplant lung function with aerosol cyclosporin.Eur Respir J. 2004;23:378–383.
Iacono AT, Smaldone GC, Keenan RJ, et al. Dose-related reserval of acute lung rejection by aerosolized cyclosporine.Am J Respir Crit Care Med. 1997;155:1690–1698.
Iacono AT, Johnson BA, Grgurich WF, et al. A randomized trial of inhaled cyclosporine in lung-transplant recipients.N Engl J Med. 2006;354:141–150.
Burckart GJ, Smaldone GC, Eldon MA, et al. Lung deposition and pharmacokinetics of cyclosporine after aerosolization in lung transplant patients.Pharm Res. 2003;20:252–256.
Le Brun PPH, Vinks AA, Touw DJ, et al. Can tobramycin inhalation be improved with a jet nebulizer?Ther Drug Monit. 1999;21:618–624.
O'Callaghan C, Barry PW. The science of nebulised drug delivery.Thorax. 1997;52:S31-S44.
Touw DJ, Jacobs FA, Brimicombe RW, Heijerman HG, Bakker W, Briemer DD. Pharmacokinetics of aerosolized tobramycin in adult patients with cystic fibrosis.Antimicrob Agents Chemother. 1997;41:184–187.
Molpeceres J, Guzman M, Bustamante P, del Rosario Aberturas M. Exothermic-endothermic heat of solution shift of cyclosporin A related to poloxamer 188 behavior in aqueous solutions.Int J Pharm. 1996;130:75–81.
Van Drooge DJ, Hinrichs WLJ, Frijlink HW. Incorporation of lipophilic drugs in sugar glasses by lyophilization using a mixture of water and tertiary butyl alcohol as solvent.J Pharm Sci. 2004;93:713–725.
Le Brun PPH, de Boer AH, Mannes GP, et al. Dry pow der inhalation of antibiotics in cystic fibrosis therapy: part 2. Inhalation of a novel colistin dry powder formulation: a feasibility study in healthy volunteers and patients.Eur J Pharm Biopharm. 2002;54:25–32.
Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions.Eur J Pharm Biopharm. 2000;50:47–60.
Sethia S, Squillante E. Solid dispersions: revival with greater possibilities and applications in oral drug delivery.Crit Rev Ther Drug Carrier Syst. 2003;20:215–247.
Kaushal AM, Gupta P, Bansal AK. Amorphous drug delivery systems: molecular aspects, design, and performance.Crit Rev Ther Drug Carrier Syst. 2004;21:133–193.
Van Drooge DJ, Hinrichs WLJ, Frijlink HW. Anomalous dissolution behaviour of tablets prepared from sugar glass-based solid dispersions.J Control Release. 2004;97:441–452.
Ericsson CH, Svartengren K, Svartengren M, et al. Repeatability of airway deposition and tracheobrounchial clearance rate over three days in chronic bronchitis.Eur Respir J. 1995;8:1886–1893.
Moller W, Haussinger K, Winkler-Heil R, et al. Mucociliary and long-term particle clearance in the airways of healthy nonsmoker subjects.J Appl Physiol. 2004;97:2200–2206.
Johnson KA. Preparation of peptide and protein powders for inhalation.Adv Drug Deliv Rev. 1997;26:3–15.
Frijlink HW, De Boer AH. Dry powder inhalers for pulmonary drug delivery.Expert Opin Drug Deliv. 2004;1:67–86.
Maa YF, Nguyen PA, Sweeney T, Shire SJ, Hsu CC. Protein inhalation powder: spray drying vs spray freeze drying.Pharm Res. 1999;16:249–254.
van Drooge DJ, Hinrichs WL, Dickhoff BH, et al. Spray freeze drying to produce a stable Dalta(9)-tetrahydrocannabinol containing inulin-based solid dispersion powder suitable for inhalation.Eur J Pharm Sci. 2005;26:231–240.
Zijlstra GS, Hinrichs WLJ, de Boer AH, Frijlink HW. The role of particle engineering in relation to formulation and de-agglomeration principle in the development of a dry powder formulation for inhalation of cetrorelix.Eur J Pharm Sci. 2004;23:139–149.
van Drooge DJ, Hinrichs WL, Visser MR, Frijlink HW. Characterization of the molecular distribution of drugs in glassy solid dispersions at the nano-meter scale, using differential scanning calorimetry and gravimetric water vapour sorption techniques.Int J Pharm. 2006;310:220–229.
Salm P, Norris RL, Taylor PJ, Davis DE, Ravenscroft PJ. A reliable high-performance liquid chromatography assay for high-throughput routine cyclosporin A monitoring in whole blood.Ther Drug Monit. 1993;15:65–69.
Scott TA, Jr Melvin EH. Determination of dextran with anthrone.Anal Chem. 1953;25:1656–1661.
Hinrichs WLJ, Prinsen MG, Frijlink HW. Inulin glasses for the stabilization of therapeutic proteins.Int J Pharm. 2001:215:163–174.
Dong A, Huang P, Caughey WS. Protein secondary structures in water from second-derivative amide I infrared spectra.Biochemistry. 1990;29:3303–3308.
Savitzky A, Golay MJE. Smoothing and differentiation of data by simplified least squares procedures.Anal Chem. 1964;36:1627–1639.
Kendrick BS, Dong A, Allison SD, Manning MC, Carpenter JF. Quantitation of the area of overlap between second-derivative amide I infrared spectra to determine the structural similarity of a protein in different states.J Pharm Sci. 1996;85:155–158.
van de Weert M, Haris PI, Hennink WE, Crommelin DJ. Fourier transform infrared spectrom etric analysis of protein conformation: effect of sampling method and stress factors.Anal Biochem. 2001;297:160–169.
van de Weert M, Haris PI, Hennink WE, Crommelin DJ. Fourier transform in frared spectrom etric analysis of protein conformation: effect of sampling method and stress factors.Anal Biochem. 2001;297:160–169.
Ziegler J, Wachtel H. Comparison of cascade impaction and laser diffraction for particle distribution measurements.Aerosol Med. 2005;18:311–324.
de Boer AH, Hagedoorn P, Gjaltema D, Geode J, Frijlink HW. Air classifier technology (ACT) in dry powder inhalation: Part 1. Introduction of a novel force distribution concept (FDC) explaining the performance of a basic air classifier on adhesive mixtures.Int J Pharm. 2003;260:187–200.
Costantino HR, Firouzabadian L, Wu C, et al. Protein spray freeze drying. 2. Effect of formulation variables on particle size and stability.J Pharm Sci. 2002;91:388–395.
Rogers TL, Johnston KP, Williams RO, 3rd. Physical stability of micronized powders produced by spray-freezing into liquid (SFL) to enhance the dissolution of an insoluble drug.Pharm Dev Technol. 2003;8:187–197.
Molpeceres J, Aberturas MR, Guzman M. Biodegradable nanoparticles as a delivery system for cyclosporine: preparation and characterization.J Microencapsul. 2000;17:599–614.
Lee EJ, Lee SW, Choi HG, Kim CK. Bioavailability of cyclosporin A dispersed in sodium lauryl sulfate-dextrin based solid microspheres.Int J Pharm. 2001;218:125–131.
Liu C, Wu J, Shi B, Zhang Y, Gao T, Pei Y. Enhancing the bioavailability of cyclosporine a using solid dispersion containing polyoxyethylene (40) stearate.Drug Dev Ind Pharm. 2006;32:115–123.
Stevenson CL, Tan MM, Lechuga-Ballesteros D. Secondary structure of cyclosporine in a spray-dried liquid crystal by FTIR.J Pharm Sci. 2003;92:1832–1843.
Bertacche V, Pini E, Stradi R, Stratta F. Quantitative determination of amorphous cyclosporine in crystalline cyclosporine samples by Fourier transform infrared spectroscopy.J Pharm Sci. 2006;95:159–166.
European Pharmacopoeia(2.9.18) Procedure for powder inhalers Strasbourg: Council of Europe, 2005; 249–250.
Vanbever R, Mintzes JD, Wang J, et al. Formulation and physical characterization of large porous particles for inhalation.Pharm Res. 1999;16:1735–1742.
Dunbar C, Scheuch G, Sommerer K, DeLong M, Verma A, Batycky R. In vitro and in vivo dose delivery characteristics of large porous particles for inhalation.Int J Pharm. 2002:245:179–189.
Edwards DA, Ben-Jebria A, Langer R. Recent advances in pulmonary drug delivery using large, porous inhaled particles.J Appl Physiol. 1998;85:379–385.
Edwards DA, Hanes J, Caponetti G, et al. Large porous particles for pulmonary drug delivery.Science. 1997;276:1868–1871.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published: June 15, 2007
Rights and permissions
About this article
Cite this article
Zijlstra, G.S., Rijkeboer, M., van Drooge, D.J. et al. Characterization of a cyclosporine solid dispersion for inhalation. AAPS J 9, 21 (2007). https://doi.org/10.1208/aapsj0902021
Received:
Accepted:
DOI: https://doi.org/10.1208/aapsj0902021