Abstract
Background
Pathological nipple discharge (PND) commonly caused by benign diseases, but occasionally it signifies a major medical concern. Ultrasonography, in addition to mammography, is regarded as the standard imaging modality in the diagnosis of PND but their sensitivity in some cases are low, subsequently we used a contrast enhanced mammography (CEDM) as supplementary diagnostic modality in patients with PND. The purpose of our study was to investigate the diagnostic efficacy of CEDM in evaluating PND patients, added values of incorporating the CEDM in the diagnostic workup of patients with PND and to demonstrate its diagnostic significance as a predictor of malignancy in these patients as there have been few studies that have addressed the role of CEDM in the evaluation of PND.
Results
Forty seven patients with PND were enrolled in this prospective study and underwent CEDM. The CEDM had high specificity (83.2%) compared to the combined sonomammography (SM) (59.3%), as there was a decrease in the number of false positive cases detected by the CEDM (6 cases) compared to the combined SM (11 cases). Combined (SM) had a moderate degree of agreement (55%, P = 0.01) with the final diagnosis, whereas CEDM had a strong degree of agreement (75%, P < 0.001). Additionally, the combined SM reported 76.6% accuracy with an area under the curve of 0.8, whereas the CEDM had 87.2% accuracy with an area under the curve of 0.89.
Conclusions
CEDM had higher specificity, positive predictive value, and accuracy than SM in PND patients, along with its stronger agreement with the final pathology results, subsequently reduce the rate of false positive cases and the rate of recall back, making it a highly accurate malignancy predictor in those patients and can be an invaluable diagnostic imaging tool for identifying associated malignancies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
After breast pain and lumps, breast discharge is the third most common complaint [1]. It is normally caused by benign diseases, but occasionally it signifies a major medical concern, especially when it is unilateral spontaneous serous or bloody discharge from a single or many orifices and it is known as pathological nipple discharge (PND) [2]. Although benign disorders like papilloma (35–56%) and duct ectasia (6–59%) account for the majority of PND causes [3], it is important to consider the possibility of underlying malignant lesions, which account for 7–33% of cases [4]. Ultrasonography, in addition to mammography, is regarded as the standard imaging modality utilized in the diagnosis of PND. However, because the masses are too small and not necessarily connected to microcalcifications, mammography frequently returns negative results and is unable to identify the underlying lesion [5]. As a result, the sensitiviy of mammography in these situations is insufficient. Further intervention may be necessary, such as cytology, when examining PND. While cytology is an uncomplicated and painless process, it has varied sensitivity and characteristics, leading to a high false-negative rate for cancer [1].
In PND, magnetic resonance imaging (MRI) is a useful supplementary diagnostic modality, particularly when the results of the other two modalities are unclear or negative, despite a high degree of clinical suspicion. Unfortunately, access to MRI is restricted to large institutions, the procedure is costly and time-consuming, and it is contraindicated in patients with metallic implants and pacemakers. MRI has a high sensitivity rate for detecting invasive breast cancer (88–95%) and dutal carcinoma insitu (DCIS) (77–90%) associated with PND [6].
Compared to MRI, Contrast-enhanced digital mammography (CEDM) is a less expensive, quicker, and simpler imaging method that patients can tolerate. It is predicated on the increased permeability within tumor locations and the contrast enrichment brought about by recently created, growing tumor vasculature. Given these benefits, CEDM allows to assess the local extent of the disease, multifocalty, and multicentricty and it has been proven to be a viable alternative imaging method to MRI in certain patients with PND [7].
According to earlier studies, CEDM dramatically raised the positive rate, accuracy, and sensitivity for the detection of breast cancer, which decreased the recall rates [8]. The purpose of our study was to investigate the diagnostic efficacy of CEDM in evaluating PND patients, added values of incorporating the CEDM in the diagnostic workup of patients with PND and to demonstrate its diagnostic significance as a predictor of malignancy in these patients. Nevertheless, there have been few studies that have addressed the role of CEDM in the evaluation of PND.
Methods
Patients population
This prospective study was approved by our institutional ethical committee. Written informed consent was obtained from each patient for participation after receiving information about all the details of the study. This study was conducted from April 2021 to September 2023 on 47 women referred from the breast clinic of our institute.
Diagnosis was established via a core needle biopsy or open surgery (microdectectomy or major duct excision) or by cytology and routine follow-up by ultrasound (every 6 months in the first year and once in the second year) in cases of typically benign lesions, considered to be the gold standard test. Female patients who are contraindicated for contrast medium administration, those with physiological nipple discharge, females less than 30 years old, and pregnant patients were excluded from our study, otherwise, all the female complaining of PND were included in our study.
Methods
After fulfilling the clinical data, including the age, color, and laterality of the discharge, all patients breasts were examined by SM, followed by CEDM (Amulet Innovality, Digital Mammography; Fujifilm, Japan).
Contrast‑enhanced digital mammography
CEDM is performed with high-energy (HE) images that were acquired at 45–50 KVp and low-energy (LE) images obtained at 27–31 KVp after the injection of an iodinated contrast agent (Zentex; 350 mg/ mL), which was injected intravenously in the antecubital fossa at a dose of 1.5 mL/kg using an automated power injector at a flow rate of 3 mL/s, followed by a saline flush, followed by a 2-min wait before breast compression. The LE image presents the morphological information equivalent to 2-dimensional (2D) mammography, whereas the HE image displays the post–contrast enhanced lesions to evaluate tumor neovascularity.
Then, a dual-energy CEDM image in the two standard positions (mediolateral oblique, MLO, and craniocaudal, CC views) was performed. Low- and high-energy images were consecutively acquired in each view, starting with the CC view of the healthy breast, then the CC and MLO of the diseased breast, and finally the MLO of the healthy breast. Then recombined images of both views of both breasts were obtained.
Image analysis
Two breast imaging consultants with at least ten years of expertise in the field of breast imaging assessed each breast lesion found in the CEDM, and they agreed on the final diagnosis. The MRI Breast Imaging-Reporting and Data System (BI-RADS) [9], served as the basis for the image analysis, which took distribution, degree of enhancement, and lesion form into account. Finding enhancing lesions and categorizing them as mass or non-mass enhancements is the first step in the assessment process. The mass is a space-occupying lesion of three dimensions, its description should include its shape (oval, round, lobulated, or irregular), margins (circumscribed or non-circumscribed), and internal enhancement features (homogeneous, heterogeneous, ring enhancement, or dark internal septation).
The non-mass is defined as a lesion that does not occupy space, Reports have been made about its distribution (focal, linear, regional, multiregional, segmental, or diffuse) and internal enhancing pattern (homogeneous, heterogeneous, clumped, and clustered). Breast lesions were finally categorized using BI-RADS; the classification was then cross-checked with the results of the histopathology or, in some circumstances, with the results of cytology.
The diagnostic algorithm was used
In CEDM, a mass lesion must meet the following criteria in order to be classified as suggestive of malignancy and assigned a BIRADS 4 or 5: it must be lobulated or irregular in shape, non-circumscribed margin, and have heterogeneous or ring enhancement. Benign masses had a rounded or oval shape, circumscribed margin, with mild homogenous or dark septa enhancement patterns [8].
The non-mass lesion was classified as suggestive of malignancy and recorded as BIRADS 4 or 5. It had a segmental, or regional distribution, linear or ductal, and a moderate to marked degree of heterogeneous or clumped enhancement. Diffuse enhancement, or many locations displaying various homogenous ring patterns, was thought to be benign [8].
Statistical analysis
Data was collected and analyzed using SPSS (Statistical Package for the Social Sciences, version 20, IBM, and Armonk, New York). The Shapiro test was used to determine the compliance of the data to normal distribution. Quantitative data were expressed as mean ± standard deviation (SD) and compared with the Student t test. Nominal data were given as numbers (n) and percentage (%). Chi2 test was implemented on such data. The accuracy of different procedures in diagnosing the nature of nipple discharge was assessed by using the receiver operator characteristics (ROC) curve. The level of confidence was kept at 95%, and hence the P value was considered significant if < 0.05. The sample size was calculated using the Epi-Info7 software program, version 23.1.
Results
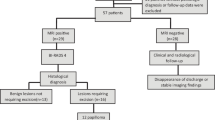
This prospective study included 47 women, whose ages ranged from 30 to 78 years, with a mean age was 45.56 ± 12.11 years, duration of discharge was 3.12 ± 2.82 months, ranging from 0.50 to 12 months. Every patient experienced spontaneous nipple discharge; 29 patients (63.8%) had uniorificial discharge, whereas 17 patients (36.2%) had multiorificial discharge. Thirteen (27.6%), fifteen (32%), and nineteen patients (40.4%) had bloody, serous, or serosanguinous discharge, respectively. Nineteen out of forty-seven (40.4%) of the women under study had a bloody discharge; eight of those cases were benign and eleven of the cases were malignant. On the other hand, out of the 28 cases (59.5%) that had a non-bloody discharge (serious and serosanguinous), 19 cases were benign and 9 cases were malignant. In 38/47 cases, the final diagnosis was made by histopathology through either a tru-cut biopsy or surgery. In the remaining 9 cases, the diagnosis was made by follow-up and cytology because the imaging results and the cytology were typical benign with stability on follow-up. with 20/47 instances (42.6%) were malignant in the form of IDC, whereas 27 cases (57.4%) of the 47 patients had benign lesions in the form of papilloma, 13 cases (27.7%), which was the most prevalent benign lesion, followed by ductectazia 10 cases (21.3%), the other pathology described in Table 1.
Sonomammographic findings
According to the results of the SM imaging, 16/47 (34%) cases were likely benign lesions and were classified as BIRADS 3, 17/47 (36.2%) cases were classified as suspicious and placed in the BIRADS 4 group, and 14/47 (29.8%) cases had malignant signs and were classified as BIRDAS 5 (Table 2).
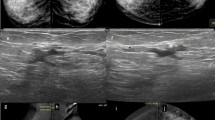
All of the BIRADS 3 patients were benign, and 11 cases out of the 17 patients classified as BIRADS 4 were false positives. Eight of the patients displayed asymmetry by mammography and dilated ducts with intraductal irregular outline hypoechoic solid masses by ultrasound; these lesions proved pathologically to be papilloma Fig. 1. Two cases were pathologically proven to be granulomatous mastitis; one of them appeared as regular dense mass lesion by mammography and as a hypoechoic mass with an regular outline by ultrasound examination Fig. 2, the second case appears as regional asymmetry by mammography and by ultrasound examination, it appears as hypoechoic non mass lesion Fig. 3. The last case was focal fibrocystic changes appeared on mammography as a focal area of asymmetry; by complementary ultrasound, there is a focal area of heterogeneity with minute cysts.
A 46-year-old female patient complaining of bloody nipple discharge from the right breast. Mammography CC (a, b) and MLO (c, d) views showed right upper outer focal asymmetry. CEDM in CC (e, f) and MLO views (g, h) showed a suspicious, right breast, clumped, non-mass enhancement of segmental distribution (BI-RADS 5). Pathology revealed intraductal papillomatosis
A 44-year-old female patient complaining of serous nipple discharge from the left breast. Mammography CC (a, b) and MLO (c, d) views showed left upper outer quadrant mass. CEDM in CC (e, f) and MLO views (g, h) showed a suspicious, left breast, moderate heterogeneous mass enhancement (BI-RADS 4). Pathology revealed granulomatous mastitis
A 39-year-old female patient complaining of bloody nipple discharge from the left breast. Mammography CC (a, b) and MLO (c, d) views showed left lower inner quadrant asymmetry. CEDM in CC (e, f) and MLO views (g, h) showed a suspicious, left breast, moderate heterogeneous regional, non-mass enhancement (BI-RADS 5). Pathology revealed granulomatous mastitis
Contrast enhanced digital mammography findings
Based on the morphology and enhancement patterns in the 47 cases, the CEDM findings were analyzed. The BI-RADS score was assigned based on the most suspicious criteria, and correlations were made with the final pathology or on follow-up (9 instances), as shown in Table 3.
All patients classified as BIRADS 2 and 3 were benign based on CEDM findings. Only one case was assigned as BIRADS 4, and it was a false positive because it showed moderate heterogeneous mass enhancement with a circumscribed margin which pathologically proved to be granulomatous mastitis Fig. 2. Among the 25 cases classified as BIRADS 5, 5 cases were found to be false positives; 3/5 cases had papillomas and displayed moderate segmental non-mass enhancement Fig. 1; 2/5 cases had moderate heterogeneous regional non-mass enhancement; granulomatous mastitis was the cause of one of these cases Fig. 3.
Finally, combined SM had a moderate degree of agreement (55%) with the final diagnosis, whereas CEDM had a strong degree of agreement (75%) with it Table 4. Then, we observed that the combined SM reported 76.6% accuracy with an area under the curve of 0.8, whereas the CEDM had 87.2% accuracy with an area under the curve of 0.89. Additionally, the CEDM had high specificity (83.2%) compared to the combined SM (59.3%), as there was a decrease in the number of false positive cases by the CEDM (6 cases) compared to the combined SM (11 cases) Table 5.
Discussion
Though PND is mostly caused by benign breast diseases, its association with malignancy risk is a serious issue, so the challenge for clinicians is to distinguish the estimated 7%-33% of breast malignancies that may present with PND from the majority of cases of nipple discharge that are secondary to a benign and sometimes unidentified cause [10]. It is still debatable whether mammography, ductography, ultrasound, MRI, or particular combinations of several modalities constitute the best radiological examination strategy, despite the use of a variety of diagnostic methods [11].
As a problem-solving tool, CEDM is generally regarded as a relatively recent imaging technique [12]. However, with regard to PND, there is a lack of literature regarding the role of CEDM in women with PND, so achieving a systematic approach in the diagnostic workup of PND was necessary. Therefore, the purpose of our study was to evaluate the accuracy of CEDM in evaluating PND patients in order to identify the underlying cause of the disease, rule out or confirm malignancy based on evaluation of its morphological criteria and enhancement patterns.
According to the findings of our investigation, the color of the discharge does not rule out malignancy. Of the 20 cases of cancer, 11/20 (55%) of the patients had bloody discharge, and 9/20 (45%) had non-bloody discharge. This conclusion was in line with a study by Fakry et al., [13] that found that bloody nipple discharge was present in 77.8% of patients with malignant lesions, but non-bloody discharge was seen in the remaining 22.2% of patients. On the other hand, we reported that 8/27 (29.6%) patients with benign lesions had bloody discharge, while 19/27 (70.3%) of them presented with non-bloody discharge, which was consistent with the results of Fakhry et al., [13] who noted that among the examined 59 cases with benign lesions 20 cases (33.8%) showed bloody discharge, and 39 cases (66.1%) showed non-bloody discharge, so the color of the discharge has no role in exclusion or confirmation of malignancy.
The frequency of malignant lesions in our study was 42.6% (20/47), which is relatively higher than the reported frequency, despite the fact that benign breast disorders are the most common cause of PND. This finding may be explained by the different nature of the study population because the study was conducted at a cancer institute. The findings of Fakhry et al., [13] were almost identical to ours, showing that out of 140 women with PND who were investigated, 81/140 (57.8%) had malignant lesions and 59/140 (42.2%) had benign lesions.
In line with our study Wang et al., [14] found that papilloma and duct ectasia were the most prevalent benign causes of PND in their study (57% and 33%, respectively), papilloma was the most common benign lesion in our study (26.7%), followed by ductectazia (21.3%). In our investigation, we stated that all malignant lesions were IDC. However, other researchers found that IDC accounted for 82.8% of malignant cases, whereas DCIS represented 17.2% [15].
By SM imaging the majority of benign lesions (59.2%) had an oval shape, well-defined margin (77.8%), and hypoechoic echopattern (48.1%); none of these lesions displayed microcalcification. In contrast, the majority of malignant lesions (70%) had irregular shapes, poorly defined margins (75%), and hypoechoic echopatterns (95%), with seven cases (35%) displaying microcalcification.
From our study, we noted that in evaluating the patients with PND, the combined SM imaging exhibited 100% sensitivity, 59.3% specificity, and 76.6% overall accuracy. Consistent with our research, Fakhry et al., [13] found that the combined SM sensitivity and specificity were 92.6% and 54.2%, respectively. Additionally, it was shown by Abdalla et al. [16] that coupled SM had an 80% sensitivity which nearly similar to our results. Most of the false positive cases in our study featured as asymmetries by mammography and dilated ducts with intraductal irregular outline hypoechoic solid masses by ultrasound; these lesions proved pathologically to be papilloma apart from one of them appear as regular mass in both SM examination and pathologically proved to be granulomatous mastitis.
The interpretation of CEDM findings in this study depends on the analysis of enhancement patterns and morphological criteria based on the MRI BIRADS lexicon. Regarding the interpretation of the mass by CEDM at our study, most of the benign lesions were rounded or oval in shape (42.8% for each), with a circumscribed margin in most of them (85.7%) and homogenous enhancement in 85.7% of the patients. However, most malignant lesions were irregular in shape (94.1%), with a non-circumscribed margin (100%) and heterogeneous enhancement (76.5%). As regard the non-mass pattern in CEDM in our study, the most common pattern in malignant lesions is linear enhancement (66.7%) followed by moderate segmental non mass enhancement (33.3%), yet theses distribution is also seen in benign lesions in our study: 50% for moderate segmental followed by moderate heterogeneous regional non mass enhancement (33.3%) and 16.7% for linear enhancement, this can explain the increased number of false positive cases in the non-mass lesion as it was 5 cases out of six false positive cases, this is in line with multiple literatures who reported that the most of the false positive cases were non mass [15] & [17].
The sensitivity and the specificity of CEDM in our study were 100% and 83.2%, respectively, with 87.2% overall accuracy. In agreement with our study as regards to the sensitivity and accuracy Fakhry et al., [13] reported 97.5% sensitivity, 79.3% accuracy of the CEDM, but they reported low specificity 54.2%, the difference in the specificity may be due to increase in the number of the false positive cases as there were 27 false positive cases out of 140 cases in their study but in our study they were 6 false positive cases out of 47 examined cases. In a retrospective study of 186 patients with negative conventional imaging results, Chung et al., [18] reported 19% positive predictive value and 63% negative predictive value for CEDM when detecting cancers and high-risk lesions in their study, however in our study CEDM had 76.9% PPV as we had only 6 false positive cases and 100%NPV as we didn’t have false negative cases.
Our study's findings indicate that CEDM, in addition to being more widely available, less expensive, and more patient-friendly than MRI, it can be used as a high-accuracy malignancy predictor as it has a strong degree of agreement with the final pathology (75%, P < 0.001) compared to the combined SM that show moderate degree of agreement (55%, P = 0.01), subsequently help us to exclude or confirm malignancy with confidence. It also has a higher specificity, PPV, accuracy and AUC in comparison to the combined SM technique, leading to reduce the rate of false positive cases that, in turn, decrease the rate of recall back, finally CEDM add a great value to the diagnostic work up of PND when incorporating in the steps of diagnosis.
This study has two primary limitations. First, a small sample size can influence the number of false positive and false negative cases, which can impair the CEDM sensitivity, specificity, PPV, and NPV. For this reason, we suggest doing numerous large-scale investigations. Second, our study was unable to compare the diagnostic performance of contrast-enhanced MRI with CEDM. As a result, we anticipate that additional research will be conducted to ascertain whether or not CEDM may be used as a substitute for contrast-enhanced MRI as a tool for problem-solving in PND.
Conclusion
Sonomammography remains a crucial imaging modality in the diagnostic workup of PND, but in certain cases, despite a patient's serious complaints, it is inconclusive. In these cases, CEDM can be an invaluable diagnostic imaging tool for identifying associated malignancies. Its higher specificity, positive predictive value, and accuracy in PND patients, along with its stronger agreement with the final pathology results, reduce the rate of false positive cases and the rate of recall back, making it a highly accurate malignancy predictor in those patients.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PND:
-
Pathological nipple discharge
- MRI:
-
Magnetic resonance imaging
- IDC:
-
Invasive ductal carcinoma
- DCIS:
-
Ductal carcinoma in situ
- CEDM:
-
Contrast-enhanced digital mammography
- SM:
-
Sonomammography
- HE:
-
High-energy
- LE:
-
Low energy
- MLO:
-
Mediolateral oblique
- CC:
-
Craniocaudal, CC
- BIRADS:
-
Breast imaging-reporting and data system
- SE:
-
Sensitivity
- SP:
-
Specificity
- PPV:
-
Positive predictive value
- NPV:
-
Negative predictive value
- AC:
-
Accuracy
- AUC:
-
Area under curve
References
Lippa N, Hurtevent-Labrot G, Ferron S, Boisserie-Lacroix M (2015) Nipple discharge: the role of imaging. Diagn Interv Imaging 96(10):1017–1032. https://doi.org/10.1016/j.diii.2015.07.004
Yılmaz R, Bender Ö, ÇelikYabul F, Dursun M, Tunacı M, Acunas G (2017) Diagnosis of nipple discharge: value of magnetic resonance imaging and ultrasonography in comparison with ductoscopy. Balkan Med J 34(2):119–126. https://doi.org/10.4274/balkanmedj.2016.0184
Gelder L, Bisschops RHC, Menke-Pluymers MBE, Westenend PJ, Plaisier PW (2015) Magnetic resonance imaging in patients with unilateral bloody nipple discharge; useful when conventional diagnostics are negative? World J Surg 39(1):184–186. https://doi.org/10.1007/s00268-014-2701-1
Lubina N et al (2015) 3.0 Tesla breast magnetic resonance imaging in patients with nipple discharge when mammography and ultrasound fail. Eur Radiol 25(5):1285–1293. https://doi.org/10.1007/s00330-014-3521-2
Manganaro L et al (2015) Breast MRI in patients with unilateral bloody and serous-bloody nipple discharge: a comparison with galactography. Biomed Res Int. https://doi.org/10.1155/2015/806368
Zaky MM, Hafez A, Zaky MM, Shoma A, Soliman NY, Elmokadem AH (2019) MRI for assessment of pathologic nipple discharge: is it mandatory? Egypt J Radiol Nucl Med. https://doi.org/10.1186/s43055-019-0105-9
Ngo M, Kim G, Phillips J, Fishman MDC, Slanetz PJ (2022) Contrast-enhanced mammography for practicing radiologists. Adv Clin Radiol 4(1):243–251. https://doi.org/10.1016/j.yacr.2022.04.012
Xing D et al (2019) Diagnostic value of contrast-enhanced spectral mammography in comparison to magnetic resonance imaging in breast lesions. J Comput Assist Tomogr 43(2):245–251. https://doi.org/10.1097/RCT.0000000000000832
Erguvan-Dogan B, Whitman GJ, Kushwaha AC, Phelps MJ, Dempsey PJ (2006) BI-RADS-MRI: a primer. AJR Am J Roentgenol 187(2):152–160. https://doi.org/10.2214/AJR.05.0572
Choi Y et al (2022) The value of adding ductography to ultrasonography for the evaluation of pathologic nipple discharge in women with negative mammography. Korean J Radiol 23(9):866–877. https://doi.org/10.3348/kjr.2021.0850
de Paula IB, Campos AM (2017) Breast imaging in patients with nipple discharge. Radiol Bras 50(6):383–388. https://doi.org/10.1590/0100-3984.2016.0103
Hegazy R, Adel L, Yasin R (2020) The value of CESM in the evaluation of intraductal breast papilloma: a comparative study with DCE-MRI. Egypt J Radiol Nucl Med. https://doi.org/10.1186/s43055-019-0122-8
Fakhry S, Abdel Rahman RW, Shaalan HS, Hassan MHI, Tealab SH, Sayed SB (2022) The added role of contrast-enhanced spectral mammography in the evaluation of pathological nipple discharge. Egypt J Radiol Nucl Med. https://doi.org/10.1186/s43055-022-00766-4
Wang LJ, Wu P, Li XX, Luo R, Bin Wang D, Bin Guan W (2018) Magnetic resonance imaging features for differentiating breast papilloma with high-risk or malignant lesions from benign papilloma: a retrospective study on 158 patients. World J Surg Oncol 16(1):1–10. https://doi.org/10.1186/s12957-018-1537-9
Filipe MD, Patuleia SIS, de Jong VMT, Vriens MR, van Diest PJ, Witkamp AJ (2020) Network meta-analysis for the diagnostic approach to pathologic nipple discharge. Clin Breast Cancer 20(6):e723–e748. https://doi.org/10.1016/j.clbc.2020.05.015
Abdalla S, Savag L, Masannat Y, Pinder SE, Fentiman IS, Hamed H (2014) Pathological nipple discharge. Open Access J Sci Technol. https://doi.org/10.11131/2014/101037
Panzironi G, Pediconi F, Sardanelli F (2019) Nipple discharge: the state of the art. BJR|Open 1(1):20180016. https://doi.org/10.1259/bjro.20180016
Chung HL et al (2022) Nipple discharge imaging evaluation with mammography, ultrasound, galactography, and MRI. Acad Radiol. https://doi.org/10.1016/j.acra.2022.05.013
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial or not for the profit sectors.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design, Material preparation, data collection and analysis were performed by all authors, and all authors wrote and commented on the first draft of the manuscript and all of them read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This prosepective study was approved by the Research Ethics Committee of the south Egypt cancer institute at Assiut University in Egypt in. Written informed consent was obtained from each patient after receiving information about the details of the study.
Consent for publication
All patients included in this research gave written informed consent to publish the data contained within this study.
Competing interests
The authors whose names are listed on the title page and shared in the Manuscript entitled: “Contrast enhanced digital mammography as a predictor of breast cancer in patient with pathological nipple discharge”, certified that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria, educational grants, participation in speakers, membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Guarantor
The scientific guarantor of this publication is Dr. Lamiaa M. R. Khalaf.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khalaf, L.M.R., El-Sharkawy, M.A.M., Zedan, M. et al. Contrast enhanced digital mammography as a predictor of breast cancer in patient with pathological nipple discharge. Egypt J Radiol Nucl Med 55, 126 (2024). https://doi.org/10.1186/s43055-024-01296-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-024-01296-x