Abstract
Background
Atrial fibrillation (AF) is characterized by the absence of p-waves on ECG and irregular rhythm. It often presents with palpitations either palpitations may occur acutely over a short period or intermittently over several years. Other cardinal symptoms of atrial fibrillation include fatigue, dyspnea, and lightheadedness; it is important however to note that most affected individuals are asymptomatic. Concurrently, sleep disorders such as obstructive sleep apnea (OSA), insomnia, narcolepsy, and circadian rhythm disorders which are a group of conditions associated with the body’s internal clock that affect the timing of sleep and alertness, are raising concerns due to their potential associations to arrhythmias. This review explores the bidirectional relationship between AF and sleep disorders, highlighting their implications for risk stratification and management strategies.
Main body
The narrative approach of this review synthesizes evidence from numerous studies obtained through meticulous literature searches. Specific sleep disorders with a bidirectional relationship with AF are the focus, with scrutiny on the prevalence of this connection. The examination delves into the pathophysiology of sleep-related autonomic dysregulation and inflammation, emphasizing potential management modalities. Various meta-analysis cohorts have highlighted a strong connection between sleep disorders and atrial fibrillation (AF). Patients with sleep disorders, especially OSA, have a higher likelihood of developing AF, and conversely, those with AF are more prone to sleep disorders. This impact is not limited to development, as sleep disorders also contribute to the progression of AF, with AF, in turn, negatively impacting sleep duration and quality. Sleep disorders may play an important role in atrial remodeling as well as electrophysiological abnormalities, rendering the atrial tissue more susceptible to arrhythmogenesis. The narrative review suggests that treating sleep disorders could not only improve sleep quality but also reduce risk factors associated with atrial fibrillation. The effective management of sleep disorders emerges as a potential challenge in preventing and treating atrial fibrillation.
Conclusion
In conclusion, this narrative study highlights the bidirectional relationship between sleep disorders and atrial fibrillation. There is a positive correlation, affecting the development, progression, and management of atrial fibrillation. The detrimental impact of sleep disorders on atrial remodeling and electrophysiological abnormalities underscores the significance of their diagnosis and treatment. Education about the importance of sleep and the benefits of sleep disorder treatment becomes imperative for patients with AF and sleep disorders.
Similar content being viewed by others
Background
Sleep disorders are a group of conditions that disturb normal sleep patterns [1]. Sleep disorders can be caused by mental health disorders, stress, irregular sleep schedules, medications as well as lifestyle habits. The International Classification of Sleep Disorders (ICSD) helps provide a standardized classification for sleep disorders [2]. These are insomnia, sleep-disordered breathing, central disorders of hypersomnolence, circadian rhythm sleep–wake disorders, parasomnias, and sleep-related movement disorders. The prevalence varies based on the specific condition. These sleep problems can be acute or chronic and affect both the pediatric and adult populations.
In the USA, studies have shown that the prevalence of sleep disorders in the general population ranges from 20 to 41.7% [3].
It can be noted that across the world, the most common sleep disorder is insomnia, followed by sleep apnea [4]. Several studies have shown that insomnia generally tends to be higher in females, while sleep-disordered breathing is higher in the elderly.
Atrial fibrillation is the most common type of cardiac arrhythmia [5]. The current global prevalence of atrial fibrillation is approximately 60 million cases and is expected to increase in the coming years [6].
There are numerous risk factors predisposing to the occurrence of atrial fibrillation ranging from hypertension, and endocrine disorders such as hyperthyroidism, and diabetes to neurologic disorders such as stroke. Lifestyle habits that increase the risk of atrial fibrillation include increased alcohol consumption and a sedentary lifestyle. Sleep disorders also have a causal relationship with atrial fibrillation. It is highly associated with obstructive and central sleep apnea [7].
Notably, patients with sleep apnea have four times the risk of developing AF as over time the sleep apnea leads to the onset of risk factors such as hypertension and diabetes known to cause AF [8]. The prevalence of atrial fibrillation increases with age and more than 1/3 of patients are > 80 years old [9].
The incidence of AF is 88% higher in patients with OSA however that of OSA in AF patients cannot be ascertained as it tends to be severely underdiagnosed in these cases [10].
OSA and AF share many common risk factors encouraging the bidirectional relationship between the two [9]. This overlap of risk factors can also cloud the direct causal relationship between the two. The need to further explore their bidirectional goes beyond atrial fibrillation to include stroke and other cardiovascular comorbidities.
Methodology
To conduct this review, a literature search was performed across key databases, including PubMed, MEDLINE, Embase, Scopus, and Cochrane Library. The search utilized a combination of Medical Subject Headings (MeSH) terms and relevant keywords focusing on atrial fibrillation, sleep disorders, and sleep-disorder-related complications. Inclusion criteria encompassed studies published in English, spanning from the inception of databases to the present. The review included observational studies, randomized controlled trials, systematic reviews, meta-analyses, and case reports addressing the relationship between atrial fibrillation and sleep disorders. Exclusion criteria involved studies not pertinent to the review's scope or those lacking crucial information on outcomes, management, and safety. Data extraction involved gathering pertinent information from selected studies, including study design, sample size, participant characteristics, intervention details, outcomes, and follow-up duration.
Main text
Epidemiology of sleep disorders and atrial fibrillation
Sleep-disordered breathing (SDB) is commonly seen in individuals with atrial fibrillation [11]. The use of polygraphy on 579 patients with paroxysmal AF to test for sleep-disordered breathing yielded significant results [12]. In North America, atrial fibrillation (AF) is the most prevalent chronic arrhythmia, impacting 0.4% of the overall population [13]. Substantial correlations have been shown in earlier research between sleep apnea and AF in patients with congestive heart failure. In 81 congestive heart failure patients, a study found that AF prevalence was considerably higher in individuals who also had sleep apnea than in individuals who did not [14].
Although the researchers established a link between sleep apnea and AF, it remains unclear whether AF was associated with sleep apnea in general or with obstructive sleep apnea (OSA) or central sleep apnea (CSA) specifically. In total, 450 individuals from the Toronto Rehabilitation Institute Sleep Research Laboratory who had congestive heart failure were included in a broader study that also investigated this issue [15]. In that investigation, we discovered a solid connection between AF and CSA, but not OSA. Just 12% of patients with OSA and 7.5% of patients without sleep apnea had AF, but 23% of CSA patients had it. Thus, there is a substantial correlation between AF and CSA in patients with congestive heart failure. The number of people with AF in the USA only is estimated to be between 3 and 6 million, and by 2050, it is expected to rise between 6 and 16 million [16, 17].
In 2010, approximately 9 million people over the age of 55 in Europe had atrial fibrillation (AF), which is about half the population of New York. It is predicted that by 2060, this number will rise to 14 million [18, 19]. Additionally, it was projected that by 2050, about 72 million people in Asia will be diagnosed with AF, which is roughly twice the population of California. Of those cases, approximately 3 million are expected to be associated with strokes [20].
Among patients suffering from OSA, AF may be triggered by intermittent hypoxemia, hypercapnia, chemoreceptor excitation, significantly elevated sympathetic drive, and increased blood pressure. These symptoms may occur nocturnally over a long time if left untreated [21]. Arrhythmogenicity can result from both hypoxemia and hypercapnia [22, 23]. Patients with OSA experience persistent nocturnal elevations in sympathetic activation during wakefulness [24], and AF is linked to elevated sympathetic drive [25, 26]. There are noticeable changes in the size of the cardiac chamber and transmural pressures that occur because of the vigorous ventilatory attempts made to clear the upper airway obstruction during apneas [27, 28].
The stimulation of stretch-activated atrial ion channels by these abrupt structural alterations may facilitate AF [29]. Furthermore, increased levels of C-reactive protein and other markers of systemic inflammation are significantly linked to the severity of OSA [30]. Consequently, there is a clear correlation between rising AF load and C-reactive protein [31]. Interestingly, AF and OSA have a lot of similar risk factors [9]. Thus, common risk factors such as obesity and CVD may be the cause of the link between OSA and AF. This has led to ongoing discussion on whether there is a direct causal relationship between OSA and AF. This is because there is a close correlation between CVD and both disorders. A higher body mass index (BMI) or obesity, advanced age, diabetes, hypertension, smoking, dyslipidemia, and male gender are among the risk factors for these two illnesses [9, 32,33,34]. According to a recent meta-analysis, ambulatory systolic blood pressure (BP) is a more accurate predictor of future atrial fibrillation (AF) than clinic BP. It was also found that 24 h, daytime, and nighttime systolic BP is all comparable with future AF [35]. Although the exact mechanism underlying how hypertension affects AF is unknown, two primary theories have been put forth.
First, hypertension can lead to hemodynamic abnormalities and an increase in left atrial pressure, which can enlarge the atrium. Secondly, autonomic dysregulation and hypertension-related activation of the renin–angiotensin–aldosterone system (RAAS) can also induce left atrial (LA) fibrosis, which can enhance the development of AF [36]. There are several pathophysiological similarities between OSA and hypertension, including increased fluid distribution, RAAS, gender, obesity, lifestyle, and poor sleep quality [32]. OSA contributes to secondary hypertension because nighttime apneas can raise average systolic and diastolic blood pressure by causing blood pressure to rise. While BMI, diabetes, smoking, dyslipidemia, and sex are known to be predictors of both OSA and AF, they were not shown to have an impact on the association between OSA and AF. There may be pathophysiological relationships between AF and OSA; however, these relationships are still theoretical. Through several pathways, having OSA may cause the onset of AF or exacerbate its consequences [37].
Negative intrathoracic pressure can occur in OSA patients [38]. Both atrial stretch and afterload may rise because of this negative pressure [9]. Consequently, there may be electrical remodeling and left ventricular hypertrophy (LVH), which raise the risk of AF [9]. Smaller refractory and action potential durations, which result from elevated vagal and sympathetic activity and cause structural and electrical remodeling in the atria, are another effect of OSA that may precipitate the onset of AF [39]. Moreover, an increase in inflammation and oxidative stress are brought on by a decrease in O2 and an increase in CO2, which may potentially aid in the onset of AF [40]. Thus, by distorting the heart's normal electrical activity, OSA may trigger or worsen AF [41,42,43,44]. According to this viewpoint, it is possible that treating OSA will help to lessen the symptoms of AF, and research data provide credence to this theory. Surprisingly, a recent meta-analysis revealed that CPAP treatment for OSA is linked to a lower risk of AF recurrence in individuals who did not have electrical cardioversion (ECV) or radiofrequency ablation (RFA) treatment [45].
Impact of sleep-related autonomic dysregulation and inflammation on atrial fibrillation
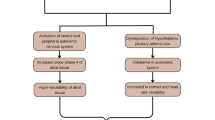
Sleep disorders have long been recognized as potential contributors to the development and progression of atrial fibrillation (AF). The connection between sleep disturbances and AF involves multifaceted pathophysiological mechanisms [46]. One such is the autonomic dysregulation caused by sleep disorders and how it plays a huge role in the pathogenesis of AF. Autonomic dysregulation has been demonstrated in several sleep disorders, including obstructive sleep apnea (OSA) and central sleep apnea (CSA) [46].
In OSA, recurrent episodes of upper airway collapse cause intermittent hypoxia and hypercapnia, thereby stimulating a hypoxic stress response by the sympathetic nervous system [47]. This response leads to sympathetic over-activity which persists during brief arousals from sleep, contributing to tachycardia and increased blood pressure [47]. Also, the repetitive apneic episodes are triggers of oxidative stress and inflammation, which can in turn cause endothelial dysfunction that leads to atrial remodeling and subsequent changes favoring AF substrate formation [48]. OSA by itself is directly linked with oxidative stress. Yamauchi et al. conducted a cross-sectional study on the connection between OSA severity and oxidative stress, using a sample size of 128 participants. Fifty individuals had severe OSA (Apnea–Hypopnea Index ≥ 30), while 70 participants had non-severe OSA (Apnea–Hypopnea Index ≤ 30). Urinary levels of 8-hydroxy-2’-deoxyguanosine, a marker of oxidative stress, were significantly elevated among patients with severe OSA [49]. OSA is associated with higher levels of interleukin-6 and tumor necrosis factor-alpha, which is known to enhance a systemic proinflammatory state [50]. This further supports the hypothesis that OSA can cause inflammation in the cardiac endothelial lining. In some animal studies, chronic intermittent hypoxia has been linked to increased atrial fibrosis, shortened refractory period, and increased AF inducibility [51].
The autonomic dysregulation caused by sleep disorders has been shown to have direct acute effects on the electrophysiology of the heart. Impaired central respiratory drive is the hallmark of CSA [52]. Transient withdrawal of parasympathetic tone during episodes of apnea leads to unopposed sympathetic activity. This consequent autonomic imbalance further suppresses vagal tone in the heart and increases sympathetic outflow, promoting a proarrhythmic environment where AF can be kick-started [53]. Hypercapnia by itself slows the velocity of conduction, further contributing to AF susceptibility [54].
Obstructive sleep apnea has been linked to atrial fibrillation development, and a critical pathophysiological mechanism behind this relationship is the concept of negative intrathoracic pressure [55]. During the episodes of upper airway collapse, there is resultant hypoxia, which the body tries to overcome by increasing negative intrathoracic pressure for adequate ventilation. This negative pressure in turn contributes to the pathogenesis of atrial fibrillation [55]. Upper airway obstruction reduces intrathoracic pressure to level below − 50 mmHg [56]. This then causes increased venous return, diminished ventricular filling, and reduced stroke volume. Thus, the circulating blood volume is restricted to the intrathoracic cavity, causing atrial dilatation. Persistent atrial dilatation causes left atrial enlargement, which may stimulate remodeling at the pulmonary vein ostia and subsequently initiate AF [57].
Baroreceptors in the intrathoracic are also activated by negative intrathoracic pressure. This causes a shortening in the refractory period due to an induction of autonomic reflex responses and a subsequent increased AF susceptibility, as demonstrated in a pig model study [58].
Contrary to the idea that increased sympathetic stimulation causes AF in OSA patients, there is a hypothesis linking other arrhythmias with increased parasympathetic activity. During an apneic episode, the vagus nerve is transiently stimulated by increased intrathoracic negative pressure, while the carotid body is stimulated by hypoxemia. Both stimuli cause temporary parasympathetic overactivity, which favors the development of bradyarrhythmias [59].
Sleep disorders as risk factors for atrial fibrillation
While good sleep is often described as being essential for good health [60] and good health is described as the absence of illness, it is no surprise that the absence or inability to have proper sleep (either in quality or duration) has been discovered to increase the risk of certain cardiac rhythm disorders such as AF [61, 62].
To understand the role of sleep disturbances in AF, we explore the role of sleep duration (with normal sleep being referenced as 8 h), sleep quality, and various sleep disorders associated with AF.
Sleep duration and atrial fibrillation
Initially, widespread consensus was that low sleep duration (< 6 h) was associated with an increased risk of developing AF [63]. However, with more studies being able to prove the correlation of both low sleep duration and excessive sleep time (> 9 h), it is now a more prevalent notion that abnormal sleep duration and timing of any kind (whether excess or little) could increase the incidence of AF in the affected population [61]. Understanding the effects of sleep duration on AF is becoming increasingly important as some studies outrightly call for the use of sleep duration and sleep quality management in the stratification of AF.
Sleep quality and atrial fibrillation
While emphasizing that short and long sleep duration may increase AF risk, we must also evaluate the increasing evidence that irregular sleep schedules and below-quality sleep can also play a role in increasing AF risk [64]. The risk of developing AF in patients with abnormal sleep quality such as mild insomnia, sleep apnea, and relative sleep disturbance was all elevated compared to the risks in individuals with normal sleep quality [65].
It is important to note that one proposed mechanism for the relationship between sleep apnea and AF is the resulting hypoxia and hypoxemia that occurs following brief breathless spells. The previous (hypoxemia) refers to reduced blood oxygen levels and the latter (hypoxia), reduced oxygen availability at the tissue level. Hypoxia triggers a cascade that activates the autonomic nervous system resulting in increased heart rate and contractility resulting in elevated vascular resistance. This compensatory mechanism now gives rise to systemic hypertension. Hypertension is a well-documented risk factor for AF [66].
Specific sleep disorder and atrial fibrillation
While various sleep disorders have been associated with atrial fibrillation, some have been more clinically researched than others. One example of this is obstructive sleep apnea (OSA). OSA is a condition in which the muscles supporting structures such as the tongue, and soft palate temporarily relax. A major risk factor for OSA is obesity [67]. In a recent meta-analysis study, OSA was found to be clinically correlated to an increase in the incidence of developing AF in the diseased population. [68]. It is important to note, however, that while these results are statistically significant, it is not yet determined at what stage of OSA is associated with the highest risk of AF.
Another disease that has been widely clinically documented is restless leg syndrome (RLS). In restless leg syndrome, patients present with an irresistible urge to move their legs or mimic leg motion. It is also commonly associated with iron deficiency [69]. RLS has been shown to increase the risk of AF in diseased populations [70]. A recent study was also able to highlight that RLS could also worsen AF severity from persistent to even permanent forms [70].
One other most recognized sleep disorder associated with AF is Narcolepsy. Narcolepsy is a disorder characterized by prolonged daytime sleepiness, cataplexy, and sleep paralysis [71]. Research has been able to show that since Narcolepsy can cause fragmented sleep, it can decrease the quality of sleep and effectively decrease sleep duration in patients, hence increasing the risk in diseased populations as compared to healthy populace [72].
Sleep and AF-related risk factors
The bidirectional relationship between both sleep disorders and AF exists in large part due to the shared comorbidities [73]. Certain comorbidities found as a result of sleep disorders are also predisposing risk factors for AF. Chief of which is hypertension. Obesity and smoking are also readily identified [66]. Another example is seen in Narcolepsy. Narcolepsy which is often associated with ANS dysfunction may in turn disrupt normal electrical activity of the heart and trigger AF [74].
Having understood the correlation between sleep disorders and AF, it is important to also emphasize that although such a connection exists, sleep disorders are still very under or misdiagnosed [75]. This could be a missing link to early risk stratification and even early initiation of preventive measures for patients with a high chance of developing AF.
Implications for risk stratification in patients with both sleep disorders and atrial fibrillation
It is critically important to stratify the risk in patients with atrial fibrillation because of the high risk of mortality and morbidity associated with it. Obstructive sleep apnea is highly associated with atrial fibrillation and affects cardiovascular risk in patients with atrial fibrillation. It has been seen that obstructive sleep apnea (OSA) is more prevalent in patients diagnosed with atrial fibrillation.
2 MACE (Major Adverse Cardiovascular events) was developed for the assessment of cardiovascular risks in patients with atrial fibrillation [76]. There is a direct relationship between the severity of OSA and 2MACE scoring. This indicates the severity of OSA is directly proportional to the increased cardiovascular risk [77].
Importance of integrated screening approaches in clinical practice
Integrated screening approaches for OSA and atrial fibrillation in clinical practice are important for several reasons. Mostly, OSA and Atrial fibrillation coexists; screening approaches help us in the early diagnosis, leading to early comprehensive care.
It also leads to improved patient outcomes, i.e., reduces cardiovascular risk in such patients. The conditions can be managed accordingly and effectively. OSA can be managed by lifestyle changes, CPAP therapy, or surgery. Atrial fibrillation is treated with anticoagulants, rhythm control strategies, or catheter ablation [78].
Integrated screening can ultimately lead to improved quality of life. This includes better sleep quality, reduced daytime fatigue, and a lower risk of stroke and other heart-related conditions [79].
Management strategies of AF in patients with sleep disorder
The management principles of atrial fibrillation commonly include rate/rhythm control and anticoagulation which is important as AF serves as a risk factor for developing other clinical conditions such as stroke and heart failure. This condition could also have an impact on the lifestyle and mental health of its sufferers. Therefore, it is important that risk factors and causes are identified and promptly tackled to give these patients a good quality of life.
In this section we look at a few sleep disorders associated with AF and how to manage AF in patients with these sleep disorders. Although sufficient data are lacking in many areas, we hope that soon, there will be certain, more effective management protocols for the AF population with sleep disorders.
Management of obstructive sleep apnea in patients with atrial fibrillation
-
1.
Continuous Positive Airway Pressure (CPAP) Therapy.
Continuous Positive Airway Pressure is the most effective treatment for moderate/severe and symptomatic OSA as it keeps the pharyngeal area from collapsing, thereby alleviating airway obstruction. In a randomized study done by Nalliah et al. to evaluate the impact of OSA management on the atrium in AF patients, it was discovered that CPAP therapy reversed atrial remodeling and this could be a potent therapy for rhythm control in this population [80]. It has also been noted that the risk of AF recurrence and progression is increased in OSA patients not using CPAP therapy. Studies have also shown that in patients who have both sleep apnea and atrial fibrillation, the recurrence rates of AF are much higher after a cardiac ablation in patients not using CPAP [81]. Untreated or under-treated sleep apnea is one of the most common reasons for recurrent atrial fibrillation after an ablation. Therefore, CPAP therapy is of great importance in this population.
-
2.
Vagus Nerve Stimulation (VNS).
A study by Malow et al. showed that VNS during sleep resulted in decreased airflow and reduced respiratory efforts which could precipitate or exacerbate apnea/hypopnea [82]. The heart is innervated by the vagus at both cardiac muscle cells and the conduction system. The effects of vagus nerve stimulation (VNS) can to some extent be predicted from its anatomical distribution. They include a reduction in heart rate (negative chronotropic action on SAN), reduction in atrioventricular conduction (negative dromotropic action on AVN), changes in threshold for induction of atrial fibrillation (AF) (action on atrial conduction system), reduction in ventricular contractility (negative inotropic action on the ventricular myocardium), reduction in threshold for induction of ventricular arrhythmias (action on ventricular conduction system) [83]. Research is still ongoing for the use of VNS in AF. However, a trial carried out by Yu et al. [84] using low-level transcutaneous electrical stimulation (LL-TS) of the auricular branch of the vagus nerve at the tragus in canines suggested that LL-TS repressed the shortening of atrial refractoriness and autonomic remodeling and protected against OSA‐induced AF. More studies need to be carried out on human models to determine if this could be a possible future management.
-
3.
Renal Denervation (RDN).
A study carried out by Xiakereti et al. [85] on beagle dog models to identify the effect of RDN on OSA-induced AF concluded that RDN reduced renal sympathetic hyperactivity and RAAS activation. It also showed that sympathetic neural remodeling of the substrate AF was downregulated, reducing AF inducibility. In the clinical setting, Witkowski et al. reported that RDN lowered blood pressure in patients with refractory HT and OSA, which was accompanied by an improvement in sleep apnea severity [86].
Based on studies that have shown that RDN could reverse the structural and sympathetic remodeling of the atrium, it is safe to say that this could become a novel modality for managing AF in OSA patients shortly.
-
4.
Low-level Baroreflex stimulation.
Baroreceptor stimulation not only modulates autonomic balance by sympathetic withdrawal but also increases vagal activation; therefore, it has been valuable in treating patients with heart failure and resistant hypertension [87]. High-frequency baroreceptor stimulation could reduce sinus rate and increase chances of AF inducibility; however, a stimulation voltage set at about 80% below the sinus rate reducing threshold can increase the atrial effective refractory period and AF threshold, thereby suppressing AF inducibility in dog models [88]. This study shows that LL-BRS but not HL-BRS could be therapeutic for AF associated with OSA.
-
5.
Beta-Blockers.
Beta-adrenergic antagonists are commonly used medications in managing AF for rate control. They also help in controlling ventricular rate and reducing the risk of mortality. It is the preferred rate control agent for patients with AF following a myocardial infarction and for patients with congestive heart failure [89].
The reported benefits of beta-blocker use in CVD patients with concomitant obstructive sleep apnea (OSA) might be limited by the thoughts of beta-blockers aggravating the apnea-induced bradycardias that are observed in these patients. However, a study negated this thought by showing that beta-blockers not only reduce apnea-induced increases in heart rate, but they do not potentiate apnea-induced HR decelerations as well as have no influence on respiratory disturbances [90]. Evidence brought by a study done on canine models of chronic OSA where metoprolol (5 mg/kg/day) was continuously administered showed inhibition of structural, sympathetic neural, and metabolic remodeling in the atria and this might potentially play a role in treating atrial remodeling, decreasing AF inducibility, and shortening the duration of AF [91]. This might be a potent and cost-effective therapy for managing AF in people with OSA; hence, future trials should be attempted on humans.
-
6.
Weight Loss.
There is a proven link between obesity and atrial fibrillation. There are multiple studies demonstrating the relationship between increasing BMI/obesity and AF. A study by Wang TJ et al. [92] showed that regardless of gender, for every 1% increase in BMI, there was a 4% increase in AF risk. Likewise, obesity is probably the most significant yet modifiable risk factor for developing obstructive sleep apnea. In most cases, weight loss may be an effective therapy for overweight/obese people with OSA; this could reduce the symptoms and sometimes lead to resolution of OSA [93]. Larger cohorts such as one conducted by Huxley et al. 2011 [94] involving 14,598 participants with a mean follow-up period of 17 years also support this, as 17.9% with atrial fibrillation was attributed to overweight and obesity.
Additionally, Tedrow et al. 2010 [95] also conducted a prospective cohort with a follow-up period of 12 years involving 34,309 female participants showed a 4.7% increase in atrial fibrillation per 1 kg/m2 increase in BMI.
Tsang et al. 2008 [96] in another prospective cohort study among 3,248 patients with a paroxysmal form of atrial fibrillation concluded that BMI predicted progression of paroxysmal to permanent AF with a hazard ratio of 1.04.
If sleep apnea is managed properly, there is a possibility that atrial fibrillation may go away. This makes it important for lifestyle modifications and weight loss strategies to be implemented in managing atrial fibrillation due to sleep disorders.
Challenges and future directions
Atrial fibrillation patients may experience sleep disorders differently than those without AF. Screening tests like sleep questionnaires and polysomnography (PSG) derived from the general medicine practice do not work well in patients with cardiovascular diseases such as atrial fibrillation. Patients with atrial fibrillation do not show the exact pathognomonic signs such as snoring or daytime sleepiness. The other symptoms such as fatigue and nocturnal dyspnea can be multifactorial such as caused by underlying comorbidities [97]. Further research is needed to evaluate sleep disorders in patients with cardiovascular diseases.
Limited access to sleep specialists; specialized sleep centers may not be readily accessible, particularly in rural or underserved areas. This can make it difficult for AF patients to receive comprehensive evaluation and treatment for sleep disorders.
Obesity is accompanied by various medical conditions such as hypertension, diabetes mellitus, and obstructive sleep apnea. Hypersthenic posture can lead to sleep apnea, which then causes AF. In this group of patients, weight loss can improve the prognosis of AF [98].
Hang Dinh Thi Dieu et al. conducted a prospective and descriptive study on 139 individuals with severe OSA with auto-adjusting CPAP at home for 6 months. The patients were followed up for over 6 months. The patients showed some side effects in the 3rd and the 6th month. The main side effects in the 3rd month were dry mouth or nose, difficulty sleeping, skin marks or rashes, and discomfort breathing. In the 6th month, some patients refused to use the CPAP due to uncomfortable feelings while sleeping [99]. However, further research is needed to improve the CPAP device without causing side effects to the patients.
Future investigations on sleep disorders in atrial fibrillation patients are on molecular and cellular levels. Neural modulation (autonomic) therapy is one of the future directions in treating OSA-associated atrial fibrillation patients [87].
Randomized control trials are needed to assess the effectiveness and duration of various sleep therapies in preventing atrial fibrillation recurrence and improving overall health.
Conclusions
This narrative study concludes sleep disorders are noted to have a bidirectional relationship with atrial fibrillation. There is a strong association between sleep disorders and atrial fibrillation patients with sleep disorders such as obstructive sleep apnea (OSA) which is more likely to develop atrial fibrillation and those with atrial fibrillation are more likely to have sleep disorders. Sleep disorders can contribute to atrial fibrillation development and progression, and atrial fibrillation can worsen the quality and duration of sleep. Patients with CPAP have proved to have side effects and worsen the quality of sleep due to the continuous usage of the device during the night.
Sleep disorders may play a major role in the atrial remodeling and electrophysiological abnormalities of the heart that contribute to atrial fibrillation. Sleep disorders may also alter the structure and function of atrial tissue which makes it more susceptible to arrhythmogenesis. This narrative review has shown that treating sleep disorders may improve sleep quality and reduce the risk factors of atrial fibrillation. Treating the sleep disorder can be an effective challenge for atrial fibrillation prevention and management.
Patients with atrial fibrillation and sleep disorders should be educated about the importance of sleep and the benefits of the treatment for sleep disorders. This narrative review also explosively emphasizes the importance of the diagnosis of sleep disorders in atrial fibrillation patients.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- AF:
-
Atrial fibrillation
- BMI:
-
Body mass index
- CSA:
-
Central sleep apnea
- CVD:
-
Cardiovascular disease
- CPAP:
-
Continuous positive airway pressure
- ECV:
-
Electrical cardioversion
- HL-BRIS:
-
High-level baroreceptor stimulation
- LL-BRS:
-
Low-level baroreceptor stimulation
- OSA:
-
Obstructive sleep apnea
- RAAS:
-
Renin angiotensin aldosterone system
- RFA:
-
Radio-frequency ablation
- SDB:
-
Sleep-disordered breathing
- VNS:
-
Vagus nerve stimulation
References
Karna B, Gupta V (2020) Sleep disorder [Internet]. PubMed. StatPearls Publishing, Treasure Island
Gauld C, Lopez R, Geoffroy PA, Morin CM, Guichard K, Giroux É, Dauvilliers Y, Dumas G, Philip P, Micoulaud-Franchi JA (2021) A systematic analysis of ICSD-3 diagnostic criteria and proposal for further structured iteration. Sleep Med Rev 1(58):101439
Hull M (2023) Sleep disorders facts & statistics | the recovery village [Internet]. The Recovery Village Drug and Alcohol Rehab. https://www.therecoveryvillage.com/mental-health/sleep-disorders/sleep-disorders-statistics/
Rodriguez JC, Dzierzewski JM, Alessi CA (2015) Sleep problems in the elderly. Med Clin N Am 99(2):431–439. https://doi.org/10.1016/j.mcna.2014.11.013
Nesheiwat Z, Goyal A, Jagtap M (2021) Atrial fibrillation [Internet]. PubMed. StatPearls Publishing, Treasure Island
Elliott AD, Middeldorp ME, Van Gelder IC, Albert CM, Sanders P (2023) Epidemiology and modifiable risk factors for atrial fibrillation. Nat Rev Cardiol 20(6):404–417. https://doi.org/10.1038/s41569-022-00820-8. Epub 2023 Jan 4. Erratum in: Nat Rev Cardiol. 2023 Jun;20(6):429. https://doi.org/10.1038/s41569-023-00834-w. PMID: 36600003
Mehra R, Benjamin EJ, Shahar E, Gottlieb DJ, Nawabit R, Kirchner HL, Sahadevan J, Redline S; Sleep Heart Health Study (2006) Association of nocturnal arrhythmias with sleep-disordered breathing: the sleep heart health study. Am J Respir Crit Care Med 173(8):910–6. https://doi.org/10.1164/rccm.200509-1442OC
Gami AS, Pressman G, Caples SM, Kanagala R, Gard JJ, Davison DE, Malouf JF, Ammash NM, Friedman PA, Somers VK (2004) Association of atrial fibrillation and obstructive sleep apnea. Circulation 110(4):364–367
Marulanda-Londono E, Chaturvedi S (2017) The interplay between obstructive sleep apnea and atrial fibrillation. Front Neurol 11(8):668
Todd K, McIntyre WF, Baranchuk A (2010) Obstructive sleep apnea and atrial fibrillation. Nat Sci Sleep 15:39–45
Krittanawong C, Liu Y, Mahtta D, Narasimhan B, Wang Z, Jneid H, Tamis-Holland JE, Mahboob A, Baber U, Mehran R, Wilson Tang WH, Ballantyne CM, Virani SS (2020) Non-traditional risk factors and the risk of myocardial infarction in the young in the US population-based cohort. Int J Cardiol Heart Vasc 30:100634
Traaen GM, Øverland B, Aakerøy L, Hunt TE, Bendz C, Sande L, Aakhus S, Zaré H, Steinshamn S, Anfinsen OG, Loennechen JP, Gullestad L, Akre H (2019) Prevalence, risk factors, and type of sleep apnea in patients with paroxysmal atrial fibrillation. Int J Cardiol Heart Vasc 26:100447
Fuster V, Ryden LE, Asinger RW et al (2001) ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: executive summary a report of the American College Of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society Of Cardiology Committee For Practice Guidelines And Policy Conferences (committee to develop guidelines for the management of patients with atrial fibrillation) developed in collaboration with the North American Society Of Pacing And Electrophysiology. Circulation 104:2118–2150
Javaheri S, Parker TJ, Liming JD et al (1998) Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation 97:2154–2159
Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS (1999) Bradley TD Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med 160:1101–1106
Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS (2006) Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 114(2):119–25. https://doi.org/10.1161/CIRCULATIONAHA.105.595140. Epub 2006 Jul 3. Erratum in: Circulation. 2006 Sep 12;114(11):e498. PMID: 16818816
Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE (2001) Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 285(18):2370–2375. https://doi.org/10.1001/jama.285.18.2370
Krijthe BP, Kunst A, Benjamin EJ, Lip GY, Franco OH, Hofman A, Witteman JC, Stricker BH, Heeringa J (2013) Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J 34(35):2746–2751. https://doi.org/10.1093/eurheartj/eht280
Di Carlo A, Bellino L, Consoli D, Mori F, Zaninelli A, Baldereschi M, Cattarinussi A, D'Alfonso MG, Gradia C, Sgherzi B, Pracucci G, Piccardi B, Polizzi B, Inzitari D; National Research Program: Progetto FAI. La Fibrillazione Atriale in Italia (2019) Prevalence of atrial fibrillation in the Italian elderly population and projections from 2020 to 2060 for Italy and the European Union: the FAI Project. Europace 21(10):1468–1475. https://doi.org/10.1093/europace/euz141
Chiang CE, Wang KL, Lip GY (2014) Stroke prevention in atrial fibrillation: an Asian perspective. Thromb Haemost 111(5):789–797. https://doi.org/10.1160/TH13-11-0948
Somers VK, Dyken ME, Clary MP et al (1995) Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 96:1897–1904
Shepard JW Jr, Garrison MW, Grither DA et al (1985) Relationship of ventricular ectopy to nocturnal oxygen desaturation in patients with chronic obstructive pulmonary disease. Am J Med 78:28–34
Rogers RM, Spear JF, Moore EN et al (1973) Vulnerability of canine ventricle to fibrillation during hypoxia and respiratory acidosis. Chest 63:986–994
Narkiewicz K, van de Borne PJ, Cooley RL et al (1998) Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation 98:772–776
Grassi G, Seravalle G, Bertinieri G et al (2003) Behaviour of the adrenergic cardiovascular drive in atrial fibrillation and cardiac arrhythmias. Acta Physiol Scand 177:399–404
Wallin BG, Delius W, Sundlof G (1974) Human muscle nerve sympathetic activity in cardiac arrhythmias. Scand J Clin Lab Invest 34:293–300
Condos WR Jr, Latham RD, Hoadley SD et al (1987) Hemodynamics of the Mueller maneuver in man: right and left heart micromanometry and Doppler echocardiography. Circulation 76:1020–1028
Hall MJ, Ando S, Floras JS et al (1998) Magnitude and time course of hemodynamic responses to Mueller maneuvers in patients with congestive heart failure. J Appl Physiol 85:1476–1484
Franz MR, Bode F (2003) Mechano-electrical feedback underlying arrhythmias: the atrial fibrillation case. Prog Biophys Mol Biol 82:163–174
Shantha G, Pelosi F, Morady F (2019) Relationship between obstructive sleep apnoea and AF. Arrhythm Electrophysiol Rev 8(3):180–183. https://doi.org/10.15420/aer.2019.35.2
Zhang L, Hou Y, Po SS (2015) Obstructive sleep apnoea and atrial fibrillation. Arrhythm Electrophysiol Rev 4(1):14–18. https://doi.org/10.15420/aer.2015.4.1.14
Bangash A, Wajid F, Poolacherla R, Mim FK, Rutkofsky IH (2020) Obstructive sleep apnea and hypertension: a review of the relationship and pathogenic association. Cureus 12(5):e8241. https://doi.org/10.7759/cureus.8241
Gumprecht J, Domek M, Lip GYH, Shantsila A (2019) Invited review: hypertension and atrial fibrillation: epidemiology, pathophysiology, and implications for management. J Hum Hypertens 33(12):824–836. https://doi.org/10.1038/s41371-019-0279-7
Wingerter R, Steiger N, Burrows A, Estes NAM 3rd (2020) Impact of lifestyle modification on atrial fibrillation. Am J Cardiol 125(2):289–297. https://doi.org/10.1016/j.amjcard.2019.10.018
Coccina F, Pierdomenico AM, De Rosa M, Cuccurullo C, Pierdomenico SD (2021) Association of clinic and ambulatory blood pressure with new-onset atrial fibrillation: a meta-analysis of observational studies. J Clin Hypertens (Greenwich) 23(6):1104–1111. https://doi.org/10.1111/jch.14256
Lau YF, Yiu KH, Siu CW, Tse HF (2012) Hypertension and atrial fibrillation: epidemiology, pathophysiology and therapeutic implications. J Hum Hypertens 26(10):563–569. https://doi.org/10.1038/jhh.2011.105
Tietjens JR, Claman D, Kezirian EJ, De Marco T, Mirzayan A, Sadroonri B, Goldberg AN, Long C, Gerstenfeld EP, Yeghiazarians Y (2019) Obstructive sleep apnea in cardiovascular disease: a review of the literature and proposed multidisciplinary clinical management strategy. J Am Heart Assoc 8(1):e010440. https://doi.org/10.1161/JAHA.118.010440
Saleeb-Mousa J, Nathanael D, Coney AM, Kalla M, Brain KL, Holmes AP (2023) Mechanisms of atrial fibrillation in obstructive sleep apnoea. Cells 12(12):1661. https://doi.org/10.3390/cells12121661
Hohl M, Linz B, Böhm M, Linz D (2014) Obstructive sleep apnea and atrial arrhythmogenesis. Curr Cardiol Rev 10(4):362–368. https://doi.org/10.2174/1573403x1004140707125137
Christou K, Moulas AN, Pastaka C, Gourgoulianis KI (2003) Antioxidant capacity in obstructive sleep apnea patients. Sleep Med 4(3):225–228. https://doi.org/10.1016/s1389-9457(02)00253-8
Shamsuzzaman AS, Winnicki M, Lanfranchi P et al (2002) Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation 105:2462–2464
Chung MK, Martin DO, Sprecher D et al (2001) C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation 104:2886–2891
Haissaguerre M, Hocini M, Denis A, Shah AJ, Komatsu Y, Yamashita S, Daly M, Amraoui S, Zellerhoff S, Picat MQ, Quotb A, Jesel L, Lim H, Ploux S, Bordachar P, Attuel G, Meillet V, Ritter P, Derval N, Sacher F, Bernus O, Cochet H, Jais P, Dubois R (2014) Driver domains in persistent atrial fibrillation. Circulation 130(7):530–538. https://doi.org/10.1161/CIRCULATIONAHA.113.005421
Sanchez AM, Germany R, Lozier MR, Schweitzer MD, Kosseifi S, Anand R (2020) Central sleep apnea and atrial fibrillation: A review on pathophysiological mechanisms and therapeutic implications. Int J Cardiol Heart Vasc 22(30):100527. https://doi.org/10.1016/j.ijcha.2020.100527
Li X, Zhou X, Xu X, Dai J, Chen C, Ma L, Li J, Mao W, Zhu M (2021) Effects of continuous positive airway pressure treatment in obstructive sleep apnea patients with atrial fibrillation: a meta-analysis. Medicine (Baltimore) 100(15):e25438. https://doi.org/10.1097/MD.0000000000025438
Greenlund IM, Carter JR (2022) Sympathetic neural responses to sleep disorders and insufficiencies. Am J Physiol Heart Circ Physiol 322(3):H337–H349
Mastino P, Rosati D, de Soccio G, Romeo MA, Pentangelo D, Venarubea S et al (2023) Oxidative stress in obstructive sleep apnea syndrome: putative pathways to hearing system impairment. Antioxidants 12(7):1430
Meliante PG, Zoccali F, Cascone F, Di Stefano V, Greco A, de Vincentiis M, Petrella C, Fiore M, Minni A, Barbato C (2023) Molecular pathology, oxidative stress, and biomarkers in obstructive sleep apnea. Int J Mol Sci 24(6):5478. https://doi.org/10.3390/ijms24065478
Kato M, Roberts-Thomson P, Phillips BG, Haynes WG, Winnicki M, Accurso V et al (2000) Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation 102(21):2607–2610
Mehra R, Redline S (2008) Sleep apnea: a proinflammatory disorder that coaggregates with obesity. J Allergy Clin Immunol 121(5):1096–1102. https://doi.org/10.1016/j.jaci.2008.04.002
Yang Y, Liu Y, Ma C, Li R, Yang Q, Zhang K, Cheng L, Yuan M, Zhang Y, Zhao Z, Li G (2022) Improving effects of eplerenone on atrial remodeling induced by chronic intermittent hypoxia in rats. Cardiovasc Pathol 60:107432. https://doi.org/10.1016/j.carpath.2022.107432
Javaheri S, Badr MS (2023) Central sleep apnea: pathophysiologic classification. Sleep 46(3):zsac113. https://doi.org/10.1093/sleep/zsac113
Iwasaki YK (2022) Mechanism and management of atrial fibrillation in the patients with obstructive sleep apnea. J Arrhythm 38(6):974–980. https://doi.org/10.1002/joa3.12784
Stevenson IH, Roberts-Thomson KC, Kistler PM, Edwards GA, Spence S, Sanders P, Kalman JM (2010) Atrial electrophysiology is altered by acute hypercapnia but not hypoxemia: implications for promotion of atrial fibrillation in pulmonary disease and sleep apnea. Heart Rhythm 7(9):1263–1270. https://doi.org/10.1016/j.hrthm.2010.03.020
Yoshihisa A, Takeishi Y (2019) Sleep disordered breathing and cardiovascular diseases. J Atheroscler Thromb 26(4):315–327. https://doi.org/10.5551/jat.RV17032
Goldberger JJ, Cain ME, Hohnloser SH, Kadish AH, Knight BP, Lauer MS, Maron BJ, Page RL, Passman RS, Siscovick D, Stevenson WG, Zipes DP; American Heart Association; American College of Cardiology Foundation; Heart Rhythm Society (2008) American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society Scientific Statement on Noninvasive Risk Stratification Techniques for Identifying Patients at Risk for Sudden Cardiac Death. A scientific statement from the American Heart Association Council on Clinical Cardiology Committee on Electrocardiography and Arrhythmias and Council on Epidemiology and Prevention. J Am Coll Cardiol 52(14):1179–99. https://doi.org/10.1016/j.jacc.2008.05.003
Walters TE, Lee G, Spence S, Larobina M, Atkinson V, Antippa P, Goldblatt J, O’Keefe M, Sanders P, Kistler PM, Kalman JM (2014) Acute atrial stretch results in conduction slowing and complex signals at the pulmonary vein to left atrial junction: insights into the mechanism of pulmonary vein arrhythmogenesis. Circ Arrhythm Electrophysiol 7(6):1189–1197. https://doi.org/10.1161/CIRCEP.114.001894
Linz D, Schotten U, Neuberger HR, Böhm M, Wirth K (2011) Negative tracheal pressure during obstructive respiratory events promotes atrial fibrillation by vagal activation. Heart Rhythm 8(9):1436–1443. https://doi.org/10.1016/j.hrthm.2011.03.053
Riaz S, Bhatti H, Sampat PJ, Dhamoon A (2020) The converging pathologies of obstructive sleep apnea and atrial arrhythmias. Cureus 12(7):e9388. https://doi.org/10.7759/cureus.9388.PMID:32754415;PMCID:PMC7386049
Irwin MR (2015) Why sleep is important for health: a psychoneuroimmunology perspective. Annu Rev Psychol 3(66):143–172. https://doi.org/10.1146/annurev-psych-010213-115205
Morovatdar N, Ebrahimi N, Rezaee R, Poorzand H, Bayat Tork MA, Sahebkar A (2019) Sleep duration and risk of atrial fibrillation: a systematic review. J Atr Fibrillation 11(6):2132. https://doi.org/10.4022/jafib.2132
Tavares L, Lador A, Valderrábano M (2021) Sleep apnea and atrial fibrillation: role of the cardiac autonomic nervous system. Methodist Debakey Cardiovasc J 17(1):49–52. https://doi.org/10.14797/ZYUT2951
Genuardi MV, Ogilvie RP, Saand AR, DeSensi RS, Saul MI, Magnani JW, Patel SR (2019) Association of short sleep duration and atrial fibrillation. Chest 156(3):544–552. https://doi.org/10.1016/j.chest.2019.01.033
Arafa A, Kokubo Y, Shimamoto K, Kashima R, Watanabe E, Sakai Y, Li J, Teramoto M, Sheerah HA, Kusano K (2022) Sleep duration and atrial fibrillation risk in the context of predictive, preventive, and personalized medicine: the Suita Study and meta-analysis of prospective cohort studies. EPMA J 13(1):77–86. https://doi.org/10.1007/s13167-022-00275-4
Chen CC, Lin CH, Yang TY, Wang TJ, Li SJ, Fang YA, Chen TJ, Tzeng HE, Chiu CC, Hao WR, Lu MY, Liu JC (2022) Association between sleep disorder and atrial fibrillation: a nationwide population-based cohort study. Sleep Med 96:50–56. https://doi.org/10.1016/j.sleep.2022.05.002
Moula AI, Parrini I, Tetta C, Lucà F, Parise G, Rao CM, Mauro E, Parise O, Matteucci F, Gulizia MM, La Meir M, Gelsomino S (2022) Obstructive sleep apnea and atrial fibrillation. J Clin Med 11(5):1242. https://doi.org/10.3390/jcm11051242
Kirsch DB (2020) Obstructive sleep apnea. Continuum (Minneap Minn) 26(4):908–928. https://doi.org/10.1212/CON.0000000000000885
Youssef I, Kamran H, Yacoub M, Patel N, Goulbourne C, Kumar S, Kane J, Hoffner H, Salifu M, McFarlane SI (2018) Obstructive sleep apnea as a risk factor for atrial fibrillation: a meta-analysis. J Sleep Disord Ther 7(1):282. https://doi.org/10.4172/2167-0277.1000282
Allen RP (2015) Restless leg syndrome/Willis-Ekbom disease pathophysiology. Sleep Med Clin 10(3):207–14, xi. https://doi.org/10.1016/j.jsmc.2015.05.022
Mirza M, Shen WK, Sofi A, Tran C, Jahangir A, Sultan S, Khan U, Viqar M, Cho C, Jahangir A (2013) Frequent periodic leg movement during sleep is an unrecognized risk factor for progression of atrial fibrillation. PLoS ONE 8(10):e78359. https://doi.org/10.1371/journal.pone.0078359
Slowik JM, Collen JF, Yow AG (2020) Narcolepsy [Internet]. PubMed. StatPearls Publishing, Treasure Island
Ben-Joseph RH, Saad R, Black J, Dabrowski EC, Taylor B, Gallucci S, Somers VK (2023) Cardiovascular burden of narcolepsy disease (CV-BOND): a real-world evidence study. Sleep 46(10):161. https://doi.org/10.1093/sleep/zsad161
Shamloo AS, Dagres N, Arya A, Hindricks G (2019) Atrial fibrillation: a review of modifiable risk factors and preventive strategies. Rom J Intern Med 57(2):99–109. https://doi.org/10.2478/rjim-2018-0045
Plazzi G, Moghadam KK, Maggi LS, Donadio V, Vetrugno R, Liguori R, Zoccoli G, Poli F, Pizza F, Pagotto U, Ferri R (2011) Autonomic disturbances in narcolepsy. Sleep Med Rev 15(3):187–196. https://doi.org/10.1016/j.smrv.2010.05.002
Stores G (2007) Clinical diagnosis and misdiagnosis of sleep disorders. J Neurol Neurosurg Psychiatry 78(12):1293–1297. https://doi.org/10.1136/jnnp.2006.111179
Platek AE, Szymanski FM, Filipiak KJ, Dudzik-Plocica A, Krzowski B, Karpinski G (2017) Stratification of cardiovascular risk in patients with atrial fibrillation and obstructive sleep apnea-validity of the 2MACE score. Sleep Breath 21(3):601–606. https://doi.org/10.1007/s11325-017-1469-6
Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P; ESC Committee for Practice Guidelines (CPG) (2012) 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 33(21):2719–2747. https://doi.org/10.1093/eurheartj/ehs253. Epub 2012 Aug 24. Erratum in: Eur Heart J. 2013 Mar;34(10):790. Erratum in: Eur Heart J. 2013 Sep;34(36):2850–1. PMID: 22922413
Faulx MD, Mehra R, Geovanini GR, Ando SI, Arzt M et al (2022) Obstructive sleep apnea and its management in patients with atrial fibrillation: an International Collaboration of Sleep Apnea Cardiovascular Trialists (INCOSACT) global survey of practicing cardiologists. IJC Heart Vasculature 42:101085. https://doi.org/10.1016/j.ijcha.2022.101085
Gahungu N, Judkins C, Gabbay E, Playford D (2019) Advances in screening for undiagnosed atrial fibrillation for stroke prevention and implications for patients with obstructive sleep apnea: a literature review and research agenda. Sleep Med 57:107–114. https://doi.org/10.1016/j.sleep.2019.01.036
Nalliah CJ, Wong GR, Lee G, Voskoboinik A, Kee K, Goldin J et al (2022) Impact of CPAP on the atrial fibrillation substrate in obstructive sleep apnea. JACC Clin Electrophysiol 8(7):869–877
Tung P, Anter E (2016) Atrial fibrillation and sleep apnea: considerations for a dual epidemic. J Atr Fibrillation 8(6):1283. https://doi.org/10.4022/jafib.1283
Malow BA, Edwards J, Marzec M, Sagher O, Fromes G (2000) Effects of vagus nerve stimulation on respiration during sleep: a pilot study. Neurology 55(10):1450–1454. https://doi.org/10.1212/wnl.55.10.1450
Capilupi MJ, Kerath SM, Becker LB (2020) Vagus nerve stimulation and the cardiovascular system. Cold Spring Harbor Perspect Med [Internet]. 10(2):a034173
Yu L, Li X, Huang B, Zhou X, Wang M, Zhou L, Meng G, Wang Y, Wang Z, Deng J, Jiang H (2017) Atrial fibrillation in acute obstructive sleep apnea: autonomic nervous mechanism and modulation. J Am Heart Assoc 6(9):e006264. https://doi.org/10.1161/JAHA.117.006264
Xiaokereti J, Guo Y, Liang X, Sun H, Li K, Zhang L, Tang B (2023) Renal denervation alleviates chronic obstructive sleep apnea-induced atrial fibrillation via inhibition of atrial fibrosis and sympathetic hyperactivity. Sleep Breath 27(5):1805–1818. https://doi.org/10.1007/s11325-023-02784-6
Witkowski A, Prejbisz A, Florczak E, Kądziela J, Śliwiński P, Bieleń P, Michałowska I, Kabat M, Warchoł E, Januszewicz M, Narkiewicz K, Somers VK, Sobotka PA, Januszewicz A (2011) Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension 58(4):559–565. https://doi.org/10.1161/HYPERTENSIONAHA.111.173799
Huang B, Liu H, Scherlag BJ, Sun L, Xing S, Xu J, Luo M, Guo Y, Cao G, Jiang H (2021) Atrial fibrillation in obstructive sleep apnea: neural mechanisms and emerging therapies. Trends Cardiovasc Med 31(2):127–132. https://doi.org/10.1016/j.tcm.2020.01.006
Liao K, Yu L, Zhou X, Saren G, Wang S, Wang Z, Huang B, Yang K, Jiang H (2015) Low-level baroreceptor stimulation suppresses atrial fibrillation by inhibiting ganglionated plexus activity. Can J Cardiol 31(6):767–774. https://doi.org/10.1016/j.cjca.2015.01.007
Fauchier L, Laborie G, Clementy N, Babuty D (2016) Beta-blockers or digoxin for atrial fibrillation and heart failure? Card Fail Rev 2(1):35–39. https://doi.org/10.15420/cfr.2015:28:2
Wolf J, Drozdowski J, Czechowicz K, Winklewski PJ, Jassem E, Kara T, Somers VK, Narkiewicz K (2016) Effect of beta-blocker therapy on heart rate response in patients with hypertension and newly diagnosed untreated obstructive sleep apnea syndrome. Int J Cardiol 1(202):67–72. https://doi.org/10.1016/j.ijcard.2015.08.139
Sun L, Yan S, Wang X, Zhao S, Li H, Wang Y, Lu S, Dong X, Zhao J, Yu S, Li M, Li Y (2017) Metoprolol prevents chronic obstructive sleep apnea-induced atrial fibrillation by inhibiting structural, sympathetic nervous and metabolic remodeling of the atria. Sci Rep 7(1):14941. https://doi.org/10.1038/s41598-017-14960-2.PMID:29097705;PMCID:PMC5668297
Wang TJ, Parise H, Levy D, D’Agostino RB Sr, Wolf PA, Vasan RS, Benjamin EJ (2004) Obesity and the risk of new-onset atrial fibrillation. JAMA 292(20):2471–2477. https://doi.org/10.1001/jama.292.20.2471
Romero-Corral A, Caples SM, Lopez-Jimenez F, Somers VK (2010) Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest 137(3):711–719. https://doi.org/10.1378/chest.09-0360.PMID:20202954;PMCID:PMC3021364
Huxley RR, Lopez FL, Folsom AR et al (2011) Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the atherosclerosis risk in communities (ARIC) study. Circulation 123:1501–1508. https://doi.org/10.1161/CIRCULATIONAHA.110.009035
Tedrow UB, Conen D, Ridker PM et al (2010) The long- and short-term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (women’s health study). J Am Coll Cardiol 55:2319–2327. https://doi.org/10.1016/j.jacc.2010.02.029
Tsang TS, Barnes ME, Miyasaka Y et al (2008) Obesity as a risk factor for the progression of paroxysmal to permanent atrial fibrillation: a longitudinal cohort study of 21 years. Eur Heart J 29:2227–2233. https://doi.org/10.1093/eurheartj/ehn324
May AM, Wang L, Kwon DH, Van Wagoner DR, Chung MK, Dalton JE, Mehra R Sleep apnea screening instrument evaluation and novel model development and validation in the paroxysmal atrial fibrillation population. 2352–9067/Published by Elsevier B.V. https://doi.org/10.1016/j.ijcha.2020.100624
Benjamin EJ, Al-Khatib SM, Desvigne-Nickens P, Alonso A, Djoussé L, Forman DE et al (2021) Research priorities in the secondary prevention of atrial fibrillation: a national heart, lung, and blood institute virtual workshop report. J Am Heart Assoc 10:e021566. https://doi.org/10.1161/JAHA.121.021566
Dinh-Thi-Dieu H, Vo-Thi-Kim A, Tran-Van H, Duong-Quy S (2020) Efficacy and adherence of auto-CPAP therapy in patients with obstructive sleep apnea: a prospective study. Multidiscip Respir Med 15(1):468. https://doi.org/10.4081/mrm.2020.468
Acknowledgements
None.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
IJO conceptualized the study; all authors were involved in the literature review; IJO and MA extracted the data from the reviewed studies; all authors wrote the final and first drafts. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ogieuhi, I.J., Ugiomoh, O.MA., Awe, M. et al. Exploring the bidirectional relationship between sleep disorders and atrial fibrillation: implications for risk stratification and management. Egypt Heart J 76, 95 (2024). https://doi.org/10.1186/s43044-024-00524-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43044-024-00524-z