Abstract
Granulocyte-macrophage colony-stimulating factor (GM-CSF) has been known as a hematopoietic growth factor and immune modulator. Recent studies revealed that GM-CSF also had pro-inflammatory functions and contributed to the pathogenicity of Th17 cells in the development of Th17-mediated autoimmune diseases. GM-CSF inhibition in some animal models of autoimmune diseases showed significant beneficial effects. Therefore, several agents targeting GM-CSF are being developed and are expected to be a useful strategy for the treatment of autoimmune diseases. Particularly, in clinical trials for rheumatoid arthritis (RA) patients, GM-CSF inhibition showed rapid and significant efficacy with no serious side effects. This article summarizes recent findings of GM-CSF and information of clinical trials targeting GM-CSF in autoimmune diseases.
Similar content being viewed by others
Background
Granulocyte-macrophage colony-stimulating factor (GM-CSF) was originally defined by its ability in vivo to generate colonies of both granulocytes and macrophages from bone marrow precursors [1]. It has also been shown to act on mature myeloid cells as pro-survival, activation, and differentiation factors [2]. Recent studies suggest that GM-CSF also has many pro-inflammatory functions and plays critical roles in the development of autoimmune and inflammatory diseases [3, 4].
Function of GM-CSF
Myeloid cell
GM-CSF promotes the survival and activation of macrophages, neutrophils, and eosinophils, as well as dendritic cell (DC) maturation [2]. On the other hand, GM-CSF-deficient mice have relatively normal myelopoiesis with abnormal lung histology that is indistinguishable from human pulmonary alveolar proteinosis (PAP) [5], indicating a redundant role of GM-CSF in myeloid cell development and its differentiation and critical roles in the maturation and surfactant catabolism of alveolar macrophages [6]. In addition to these functions, GM-CSF is reported to have diverse functions on mature myeloid cells, including enhancement of pro-inflammatory cytokine production [7], antigen presentation [8], induction of phagocytosis [9–11], and promotion of leukocyte chemotaxis and adhesion [12, 13].
GM-CSF can polarize macrophages into M1-like inflammatory macrophages, which produce a variety of inflammatory cytokines such as TNF, IL-6, IL-12p70, IL-23, or IL-1β, and thus promote Th1-Th17 responses [7, 14, 15]. On the other hand, the association of GM-CSF and Th2 immunity is also reported in allergic airway inflammation [16, 17].
GM-CSF positively regulates the development of dermal migratory CD103+CD11b− and gut migratory CD103+CD11b+ DCs [18, 19] but negatively regulates the development of plasmacytoid DCs (pDCs) [20] and resident CD8+ DCs [19]. GM-CSF is also reported to induce the development of inflammatory monocyte-derived DCs (moDCs) in vitro [21], but its effect in vivo has not been established well. It was reported that GM-CSF transgenic mice have increased the number of moDCs [22] and GM-CSF-deficient mice with inflammatory arthritis have markedly reduced the number of moDCs [23]. On the other hand, in the other reports, GM-CSF was shown to be dispensable for the differentiation of moDCs, at least during acute infections [19, 24].
In neutrophils, GM-CSF upregulates the antimicrobial functions such as phagocytosis, reactive oxygen species (ROS) production, or expression of the integrin CD11b which increases cellular adhesion and tissue entry [12, 25].
The effect of GM-CSF on osteoclast differentiation is quite complex, for it has both enhancing and suppressive actions. Under the steady state, osteoclasts are known to differentiate from hematopoietic precursors of the monocyte/macrophage lineage in the presence of M-CSF and receptor activator of NFκB ligand (RANKL) [26]. GM-CSF induces shedding of M-CSF receptor, resulting in disruption of osteoclast differentiation [27]. On the other hand, the differentiation of osteoclast precursors generated in the presence of GM-CSF or GM-CSF plus TNFα was not inhibited by GM-CSF in vitro, indicating that a different set of osteoclast precursors is available in inflammatory arthritis and that they respond to a variety of pro-inflammatory cytokines which compensate for the loss of M-CSF signaling [28, 29]. GM-CSF is also reported to induce fusion of prefusion osteoclasts to form the bone-resorbing osteoclasts and induce bone erosion [30]. Conversely, other report suggested that GM-CSF inhibited the resorption ability of osteoclasts, indicating the existence of another osteoclastic pathway [28].
B cell
Among B cells, innate response activator (IRA) B cells, a B1a B cell-derived inflammatory subset, produce GM-CSF and also express GM-CSF receptors [31, 32]. GM-CSF controls IgM production from IRA B cells in an autocrine manner which is essential to protect from bacterial infection [31, 32].
Neuron
Sensory nerves express GM-CSF receptors, and GM-CSF is reported as a key mediator in bone-cancer pain [33], osteoarthritis pain, and inflammatory arthritic pain [34, 35]. A sensory nerve-specific knockdown of GM-CSF receptors attenuated tumor-evoked pain [33]. GM-CSF deficiency or neutralization also abolished osteoarthritis pain and inflammatory arthritic pain [34, 35].
GM-CSF receptor
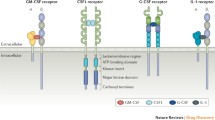
GM-CSF receptor consists of an α-subunit which binds GM-CSF with low affinity (GMRα) and a signal-transducing βc-subunit which is shared with the IL-3 and IL-5 receptors [36]. The binary complex of GM-CSF and GMRα interacts with a free βc-subunit and forms the high-affinity hexamer complex [37]. Dodecamer complexes formed by lateral aggregation of two hexamer complexes enable Jak2 associated with a βc-subunit to dimerize and transphosphorylate, but the hexamer complexes do not [38]. This structure leads to dose-dependent responses of GM-CSF receptor activation. Low concentration of GM-CSF, as in normal condition, causes βc Ser585 phosphorylation and activates 14-3-3/PI-3 kinase pathway which only leads to cell survival. Higher concentration of GM-CSF, as in inflammatory condition, turns off βc Ser585 phosphorylation and mediated βc Tyr577 phosphorylation and activation of Jak2/STAT5 pathway, Ras/mitogen-activated protein kinase pathway, and PI-3 kinase pathway, resulting in promotion of cell survival, proliferation, and activation [37].
The membrane-bound GM-CSF receptor is expressed on myeloid cells [39] and on some non-myeloid cells, such as epithelial cells [40], endothelial cells [41], and neurons [33]. There also exists a soluble GM-CSF receptor alpha subunit [42]. The function of this soluble GM-CSF receptor is unclear, but it may be required to inhibit ligand binding to cells which express membrane-bound GM-CSF receptors [43].
Production of GM-CSF
A wide variety of cells can produce GM-CSF. Major sources of GM-CSF are T and B cells, monocyte/macrophage endothelial cells, and fibroblasts. Neutrophils, eosinophils, epithelial cells, mesothelial cells, Paneth cells, chondrocytes, and tumor cells can also produce GM-CSF [44]. The production of GM-CSF is stimulated by various factors, including TNF, IL-1, toll-like receptor agonists, and prostaglandin E2 [45, 46]. Recently, the pathogenicity of GM-CSF-producing CD4 T cells in autoimmune and inflammatory diseases is clarified and gaining increasing attention [3, 4].
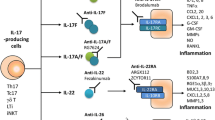
Recently, Th17 cells were clarified to have high plasticity [47]. The “classical” Th17 cells driven by transforming growth factor-β1 (TGFβ1) and IL-6 have been reported to be weak inducers of inflammation [48, 49]. Conversely, IL-23 together with IL-1β induces the differentiation of highly pathogenic Th17 cells (Th1/17 cells) which also express CXCR3 and T-bet and produce IL-17, IFN-γ, and GM-CSF in mice [48, 49]. Recent studies clarified the production of GM-CSF is critical for the pro-inflammatory function of Th17 cells [3, 4]. In humans, IL-12, instead of IL-23, together with IL-1β is reported to promote the differentiation of Th1/17 cells [50]. Th1/17 cells can be distinguished from Th1 cells by the expression of CD161, a hallmark of Th17 progeny cells in humans [51]. A recent study reported that IL-23 drives switch of surface signature from CCR6 to CCR2 which defines GM-CSF/IFNγ-producing inflammatory Th17 cells and that CCR2 drives these cells to the central nervous system (CNS) in experimental autoimmune encephalomyelitis (EAE) [52]. The pathway to induce GM-CSF production in Th cells has not been clarified well yet. T-bet was reported to drive CCR6−CCR2+ GM-CSF/IFNγ-producing Th17 cell formation [52]. On the other hand, T-bet-deficient Th17 cells are reported to have normal GM-CSF production [3]. Ectopic RORγt expression showed that RORγt drove GM-CSF production in Th cells [4]. Conversely, RORγt-deficient CD4 T cells were also able to produce GM-CSF [3]. These reports indicate the existence of additional pathways.
GM-CSF is also reported to be produced by Th1 cells and is crucial for their encephalitogenicity [4]. It was reported that STAT4 regulated GM-CSF production in Th1 cells but not in Th17 cells [53]. On the other hand, the other report indicated that STAT4 regulated GM-CSF production in both Th1 and Th17 cells by directly binding to the Csf2 promoter [54]. Recent findings on Th17 plasticity and heterogeneity indicate that it is necessary to re-examine previous studies in this field.
In addition to these cells, recent studies reported the existence of an IL-2- or IL-7-activated STAT5-dependent novel subset of GM-CSF-producing Th cells (Th-GM) which express low or undetectable T-bet, GATA-3, or RORγt [55, 56] and that Th-GM cells were able to induce more severe EAE than Th17 or Th1 cells [55]. In humans, the CCR10+CCR4+CXCR3−CCR6− signature was reported to define Th-GM [56]. It is possible that Th-GM cooperate with Th1/17 cells or Th1 cells to exacerbate the development of inflammation.
Th2 cells are also reported as one of the GM-CSF-producing cells [57]. A positive correlation between GATA-3+ cells and GM-CSF+ cells in the nasal mucosa of allergic rhinitis patients is reported [58]; however, the precise mechanism of GM-CSF production in Th2 cells has not been analyzed yet.
GM-CSF in autoimmune disease
Recent evidence revealed that GM-CSF played critical roles in the development of many autoimmune diseases. GM-CSF depletion or neutralization suppresses many autoimmune disease models, including EAE [3, 4], arthritis [59–61], arthritis-related interstitial lung disease [60], nephritis [62], or psoriasis [63]. On the other hand, GM-CSF administration is reported to improve the models of myasthenia gravis [64], type 1 diabetes [65], or colitis [66].
GM-CSF in the CNS
IL-17-producing Th17 cells have been reported as central mediators of CNS inflammation in both EAE and multiple sclerosis (MS) [67, 68]. However, recent studies reported that GM-CSF was essential for the encephalitogenicity of CD4 T cells in EAE and that IL-17 was dispensable for the development of EAE [3, 4]. The concentrations of GM-CSF and the number of GM-CSF-producing CD4 T cells in the cerebrospinal fluid were reported to be elevated in MS patients [56, 69]. GM-CSF deficiency or neutralization was reported to prevent the onset of EAE [70, 71]. In contrast, the administration of recombinant GM-CSF exacerbated EAE [70].
GM-CSF induces the proliferation and activation of microglial cells which produce highly neurotoxic substances such as ROS, nitrogen species, and glutamate [71, 72]. GM-CSF-producing CD4 T cells also induce the polarization of neurotoxic M1-like phenotype of microglia and promote the production of pro-inflammatory cytokines such as IL-1β, IL-6, and TNFα, which also contribute to myelin sheath damage [72, 73]. GM-CSF is also required for the recruitment of peripheral myeloid cells that contribute to blood-brain barrier and blood-spinal cord barrier disruption and demyelization into the CNS [74, 75]. These resident and infiltrating antigen-presenting cells (APCs) re-stimulate T cells and lead to further APC activation [76].
GM-CSF in arthritis
In the models of arthritis, IL-17 has been reported as a main pathogenic cytokine as in EAE [77, 78]. IL-17 deficiency ameliorated collagen-induced arthritis (CIA) but did not completely inhibit it [78]. IL-17 inhibition was also reported to be an unsatisfactory method for the treatment of rheumatoid arthritis (RA) [79]. These reports indicated the existence of the other critical factors in the development of arthritis.
In RA patients, the concentration of GM-CSF in the synovial fluid and plasma was elevated [80, 81] and the administration of recombinant GM-CSF exacerbated the disease activity [82]. Bone marrow adjacent to the RA joints contains an increased number of granulocyte-macrophage progenitors, colony-forming unit granulocyte-macrophages (CFU-GM), which can differentiate into granulocytes or macrophages with GM-CSF stimulation [83] and also into osteoclasts with M-CSF and RANKL stimulation [84]. The frequency of GM-CSF-producing T helper cells in synovial fluid cells was also significantly increased compared to peripheral blood mononuclear cells (PBMCs) and correlated with erythrocyte sedimentation rate (ESR) levels in juvenile idiopathic arthritis (JIA) [85].
In mouse models of arthritis, GM-CSF deficiency or neutralization prevented the development of arthritis [59–61] and reduced the concentrations of TNF and IL-1 in joints [59]. Conversely, GM-CSF administration exacerbated arthritis [86]. In arthritis of SKG mice, GM-CSF secreted by T cells upregulated the production of pro-inflammatory cytokines such as IL-6 or IL-1β from macrophages [60, 87]. This in turn induced further differentiation and expansion of IL-17-producing and GM-CSF-producing CD4 T cells [60] and exacerbated arthritis.
GM-CSF in arthritis-related interstitial lung disease
SKG arthritis model develops chronic-progressive interstitial lung disease (ILD) which histologically resembles connective tissue disease-associated ILD (CTD-ILD) [60, 88]. This model was characterized with massive infiltration of Th17 cells, GM-CSF-producing CD4 T cells, and neutrophils with fibrosis in the lungs [60]. The overexpression of GM-CSF was reported to induce severe neutrophil, eosinophil, and macrophage infiltration with fibrosis in the lungs [89, 90]. GM-CSF promotes macrophages to produce IL-6 and IL-1β and enhances differentiation of IL-17A and/or GM-CSF-producing T cells and therefore infiltration of neutrophils into the lungs [60]. Neutrophils were reported to produce ROS, MMPs, neutrophil elastase, or myeloperoxidase and cause parenchymal and stromal cell injury in the lungs [91–93]. GM-CSF also stimulates macrophages to release profibrotic cytokines and induces fibrosis by direct stimulation of airway smooth muscle cells [90, 94]. GM-CSF neutralization completely blocked the development of ILD in SKG mice but IL-17A neutralization did not, indicating that GM-CSF played a more critical role than IL-17A in this ILD [60].
The contribution of GM-CSF in human ILD has not been analyzed well yet. In patients with pulmonary fibrosis, the concentration of GM-CSF in the bronchoalveolar lavage fluid (BALF) was reported to be elevated [95, 96]. A recent report also reported that serum concentration of GM-CSF was associated with ILD in patients with RA [97]. Further studies to clarify the contribution of GM-CSF in CTD-ILD are awaited.
GM-CSF in the intestine
In the intestine, GM-CSF contributes to mucosal barrier function and resistance to bacterial translocation by promoting the recruitment and activation of myeloid cells. GM-CSF also promotes tissue repair via acceleration of epithelial cell proliferation and macrophages as effectors of wound healing [98–100].
Recent studies suggested that mucosal innate immunodeficiency caused by a variety of genetic defects contributed the susceptibility of Crohn’s disease (CD) and increased the translocation of pathogens to the bowel tissue [101]. Higher levels of GM-CSF secretion have been detected in mucosal lesions of inflammatory bowel disease (IBD) compared with normal mucosa [102, 103] and also in the colon lesions of dextran sodium (DSS)-induced colitis mice model [104]. On the other hand, in CD, the increased levels of GM-CSF autoantibodies have been reported [105]. The levels of GM-CSF autoantibodies correlated with the disease activity and inversely correlated with the neutrophil phagocytic activity in CD patients [105]. GM-CSF-deficient mice were reported to be more susceptible to acute DSS-induced colitis [106], and the severity of this colitis was largely prevented by GM-CSF administration [66, 107]. Conversely, GM-CSF neutralization was reported to ameliorate 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis [108] and IL-23-driven colitis [109]. The overexpression of GM-CSF in the stomach was reported to lead to autoimmune gastritis [110]. These data indicated the possibilities that both relative shortage and excessive amount of GM-CSF could induce colitis. Further studies are also needed to clarify whether GM-CSF autoantibodies in CD patients are pathogenic or not pathogenic and produced just as a result of elevated GM-CSF.
There are some trials of GM-CSF administration for the treatment of CD patients. Initial reports indicated a high rate of clinical response and remission with minimal adverse effects [111–113]. However, a recent large randomized trial reported that it is not effective for induction of clinical remission or improvement in active CD [114]. The pathogenic mechanism of CD patients is considered to be heterogeneous. Therefore, GM-CSF administration might be effective only in some subgroups of patients.
GM-CSF target therapy
There are several ongoing or completed clinical trials targeting GM-CSF or GM-CSF receptor (Table 1). Detailed information is available at ClinicalTrials.gov. Although GM-CSF inhibition showed rapid clinical response with no serious adverse reactions so far [115–117], there are some potential side effects which need to be monitored. The existence of GM-CSF autoantibodies or the mutations of GM-CSF receptor are reported to cause PAP [6]. On the other hand, healthy individuals also have GM-CSF autoantibodies [118], suggesting that the risk of PAP is increased only when GM-CSF autoantibody levels are increased above a critical threshold [119]. In addition, GM-CSF inhibition might exacerbate the existing Crohn’s disease as mentioned above. An increased susceptibility to infections in GM-CSF-deficient mice [5, 120] also indicates the risk of infection in GM-CSF target therapy.
Mavrilimumab
Mavrilimumab is a human monoclonal antibody against GM-CSF receptor α. In the first phase 1 study, 32 subjects with mild RA received single intravenous escalating doses of mavrilimumab and showed its safety and tolerability. Reductions of acute-phase reactants and disease activity score (DAS) 28 was also observed [121].
A phase 2a randomized, double-blind, placebo-controlled, ascending-dose study in subjects with moderate to severe active RA (EARTH study) reported significant efficacy with no serious adverse events [117]. In this study, 239 patients with active RA despite methotrexate (MTX) treatment received subcutaneous mavrilimumab or placebo every other week for 12 weeks on stable-background MTX therapy and 55.7 % of all mavrilimumab-treated participants met the primary end point of achieving a ≥1.2 decrease from baseline in the DAS (DAS28-CRP) vs 34.7 % of placebo-treated participants at week 12. All mavrilimumab-treated patients showed a response by week 2. The 100 mg dose of mavrilimumab demonstrated a significant effect vs placebo on DAS28-CRP <2.6, all categories of the American College of Rheumatology (ACR) criteria, and the Health Assessment Questionnaire Disability Index.
In a subsequent phase 2b study (EARTH EXPLORER 1) [122–125], 326 patients with moderate to severe RA received an ascending dose of mavrilimumab or placebo every 2 weeks plus MTX for 24 weeks and showed an acceptable safety and tolerability. A statistically significant difference in DAS28-CRP was observed in all doses of mavrilimumab vs placebo at week 12, and a significantly higher ACR response rate of mavrilimumab-treated subjects than that of placebo was observed at week 24. Particularly, the 150 mg dose showed a significant difference vs placebo for these parameters as early as week 1.
A nonrandomized, open-label phase 2 study to evaluate the long-term safety and tolerability from day 1 through to approximately 5 years is ongoing (NCT01712399) [126]. This study enrolled RA patients who had completed the EARTH EXPLORER 1 and 2 studies or were rescued as inadequate responders at a predefined time point, and they received 100 mg of mavrilimumab every other week. At week 74, mavrilimumab demonstrated sustained safety and efficacy with DAS28-CRP <3.2 and <2.5 rates of 57.3 and 38.5 %, respectively, and 68 % of patients showed no radiographic progression [127].
A randomized, double-blind, placebo-controlled phase 2 study (EARTH EXPLORER 2) to compare the safety and efficacy of mavrilimumab with those of golimumab, an anti-TNF antibody in 120 patients with moderate to severe RA who had an inadequate response to one or two anti-TNF agents, was completed [128].
MOR103
MOR103, which is a fully human monoclonal antibody against GM-CSF, has shown preliminary evidence of safety and rapid efficacy (within 2 weeks) in a randomized, double-blind, placebo-controlled, dose-escalating phase 1b/2a trial for patients with moderate RA (n = 96) [116]. Patients received four times of weekly intravenous MOR103 or placebo, and subjects receiving higher doses of MOR103 (1.0 and 1.5 mg/kg) showed significant improvement in DAS28 scores and joint counts and significantly higher European League Against Rheumatism response rates than subjects receiving placebo.
MOR103 was also tested in a randomized, double-blind, placebo-controlled phase 1b trial for patients with relapsing-remitting MS or secondary progressive MS. Patients received placebo or an escalating dose of MOR103 every 2 weeks for 10 weeks and showed acceptable tolerability of MOR103 [115].
Namilumab (MT203)
Namilumab is a human monoclonal antibody against GM-CSF. In a randomized, double-blind, dose-escalating phase 1b study, mild to moderate RA patients received three times of every 2-week injection of namilumab and showed its safety and tolerability [129]. The other trials testing namilumab is ongoing: a dose-finding phase 2 study of namilumab in combination with MTX in moderate to severe RA patients with inadequate response to MTX or one TNF inhibitor [130] and a phase 2 trial to evaluate the efficacy and safety of the combination of the existing MTX and namilumab vs adalimumab, an anti-TNF antibody in patients with moderate to severe early RA inadequately responding to MTX [131].
It is also being tested in a randomized double-blind phase 2 trial for moderate to severe plaque psoriasis [132].
KB003
KB003 is a humanized monoclonal antibody targeting GM-CSF. A randomized phase 2 study in RA patients showed safety and tolerability in 3 months of repeated dosing [133].
MORAb-002
MORAb-002 is a human monoclonal antibody against GM-CSF. A randomized, double-blind phase 1 trial in RA was completed recently [134].
Conclusions
Recent studies clarified the pivotal roles of GM-CSF in the development of many autoimmune diseases. Much attention has been focused on the inhibition of GM-CSF as an attractive approach for the treatment of these diseases. Further studies to clarify the molecular mechanism of GM-CSF production and precise role of GM-CSF in the development of autoimmune disease are awaited with interest.
Abbreviations
- APC:
-
antigen-presenting cell
- CIA:
-
collagen-induced arthritis
- CTD-ILD:
-
connective tissue disease-associated interstitial lung disease
- DAS:
-
disease activity score
- DC:
-
dendritic cell
- EAE:
-
experimental autoimmune encephalomyelitis
- GM-CSF:
-
granulocyte-macrophage colony-stimulating factor
- ILD:
-
interstitial lung disease
- MS:
-
multiple sclerosis
- MTX:
-
methotrexate
- PAP:
-
pulmonary alveolar proteinosis
- RA:
-
rheumatoid arthritis
References
Burgess AW, Camakaris J, Metcalf D. Purification and properties of colony-stimulating factor from mouse lung-conditioned medium. J Biol Chem. 1977;252:1998–2003.
Hamilton JA, Achuthan A. Colony stimulating factors and myeloid cell biology in health and disease. Trends Immunol. 2013;34:81–9.
El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, et al. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12:568–75.
Codarri L, Gyülvészi G, Tosevski V, Hesske L, Fontana A, Magnenat L, et al. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12:560–7.
Stanley E, Lieschke GJ, Grail D, Metcalf D, Hodgson G, Gall JA, et al. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci U S A. 1994;91:5592–6.
Carey B, Trapnell BC. The molecular basis of pulmonary alveolar proteinosis. Clin Immunol. 2010;135:223–35.
Fleetwood AJ, Lawrence T, Hamilton JA, Cook AD. Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: implications for CSF blockade in inflammation. J Immunol. 2007;178:5245–52.
Morrissey PJ, Bressler L, Park LS, Alpert A, Gillis S. Granulocyte-macrophage colony-stimulating factor augments the primary antibody response by enhancing the function of antigen-presenting cells. J Immunol. 1987;139:1113–9.
Berclaz PY, Zsengellér Z, Shibata Y, Otake K, Strasbaugh S, Whitsett JA, et al. Endocytic internalization of adenovirus, nonspecific phagocytosis, and cytoskeletal organization are coordinately regulated in alveolar macrophages by GM-CSF and PU.1. J Immunol. 2002;169:6332–42.
Berclaz PY, Shibata Y, Whitsett JA, Trapnell BC. GM-CSF, via PU.1, regulates alveolar macrophage Fcgamma R-mediated phagocytosis and the IL-18/IFN-gamma -mediated molecular connection between innate and adaptive immunity in the lung. Blood. 2002;100:4193–200.
Collins HL, Bancroft GJ. Cytokine enhancement of complement-dependent phagocytosis by macrophages: synergy of tumor necrosis factor-alpha and granulocyte-macrophage colony-stimulating factor for phagocytosis of Cryptococcus neoformans. Eur J Immunol. 1992;22:1447–54.
Sakagami T, Uchida K, Suzuki T, Carey BC, Wood RE, Wert SE, et al. Human GM-CSF autoantibodies and reproduction of pulmonary alveolar proteinosis. N Engl J Med. 2009;361:2679–81.
Gomez-Cambronero J, Horn J, Paul CC, Baumann MA. Granulocyte-macrophage colony-stimulating factor is a chemoattractant cytokine for human neutrophils: involvement of the ribosomal p70 S6 kinase signaling pathway. J Immunol. 2003;171:6846–55.
Verreck FA, de Boer T, Langenberg DM, Hoeve MA, Kramer M, Vaisberg E, et al. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci U S A. 2004;101:4560–5.
Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N, et al. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol. 2011;12:231–8.
Cates EC, Fattouh R, Wattie J, Inman MD, Goncharova S, Coyle AJ, et al. Intranasal exposure of mice to house dust mite elicits allergic airway inflammation via a GM-CSF-mediated mechanism. J Immunol. 2004;173:6384–92.
Willart MA, Deswarte K, Pouliot P, Braun H, Beyaert R, Lambrecht BN, et al. Interleukin-1α controls allergic sensitization to inhaled house dust mite via the epithelial release of GM-CSF and IL-33. J Exp Med. 2012;209:1505–17.
Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, et al. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–25.
Zhan Y, Xu Y, Lew AM. The regulation of the development and function of dendritic cell subsets by GM-CSF: more than a hematopoietic growth factor. Mol Immunol. 2012;52:30–7.
Esashi E, Wang YH, Perng O, Qin XF, Liu YJ, Watowich SS. The signal transducer STAT5 inhibits plasmacytoid dendritic cell development by suppressing transcription factor IRF8. Immunity. 2008;28:509–20.
Caux C, Vanbervliet B, Massacrier C, Dezutter-Dambuyant C, de Saint-Vis B, Jacquet C, et al. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+ TNF alpha. J Exp Med. 1996;184:695–706.
Zhan Y, Carrington EM, van Nieuwenhuijze A, Bedoui S, Seah S, Xu Y, et al. GM-CSF increases cross-presentation and CD103 expression by mouse CD8+ spleen dendritic cells. Eur J Immunol. 2011;41:2585–95.
Campbell IK, van Nieuwenhuijze A, Segura E, O'Donnell K, Coghill E, Hommel M, et al. Differentiation of inflammatory dendritic cells is mediated by NF-κB1-dependent GM-CSF production in CD4 T cells. J Immunol. 2011;186:5468–77.
Greter M, Helft J, Chow A, Hashimoto D, Mortha A, Agudo-Cantero J, et al. GM-CSF controls nonlymphoid tissue dendritic cell homeostasis but is dispensable for the differentiation of inflammatory dendritic cells. Immunity. 2012;36:1031–46.
Uchida K, Beck DC, Yamamoto T, Berclaz PY, Abe S, Staudt MK, et al. GM-CSF autoantibodies and neutrophil dysfunction in pulmonary alveolar proteinosis. N Engl J Med. 2007;356:567–79.
Adamopoulos IE, Mellins ED. Alternative pathways of osteoclastogenesis in inflammatory arthritis. Nat Rev Rheumatol. 2015;11:189–94.
Hiasa M, Abe M, Nakano A, Oda A, Amou H, Kido S, et al. GM-CSF and IL-4 induce dendritic cell differentiation and disrupt osteoclastogenesis through M-CSF receptor shedding by up-regulation of TNF-alpha converting enzyme (TACE). Blood. 2009;114:4517–26.
Nomura K, Kuroda S, Yoshikawa H, Tomita T. Inflammatory osteoclastogenesis can be induced by GM-CSF and activated under TNF immunity. Biochem Biophys Res Commun. 2008;367:881–7.
Rivollier A, Mazzorana M, Tebib J, Piperno M, Aitsiselmi T, Rabourdin-Combe C, et al. Immature dendritic cell transdifferentiation into osteoclasts: a novel pathway sustained by the rheumatoid arthritis microenvironment. Blood. 2004;104:4029–37.
Lee MS, Kim HS, Yeon JT, Choi SW, Chun CH, Kwak HB, et al. GM-CSF regulates fusion of mononuclear osteoclasts into bone-resorbing osteoclasts by activating the Ras/ERK pathway. J Immunol. 2009;183:3390–9.
Rauch PJ, Chudnovskiy A, Robbins CS, Weber GF, Etzrodt M, Hilgendorf I, et al. Innate response activator B cells protect against microbial sepsis. Science. 2012;335:597–601.
Weber GF, Chousterman BG, Hilgendorf I, Robbins CS, Theurl I, Gerhardt LM, et al. Pleural innate response activator B cells protect against pneumonia via a GM-CSF-IgM axis. J Exp Med. 2014;211:1243–56.
Schweizerhof M, Stösser S, Kurejova M, Njoo C, Gangadharan V, Agarwal N, et al. Hematopoietic colony-stimulating factors mediate tumor-nerve interactions and bone cancer pain. Nat Med. 2009;15:802–7.
Cook AD, Pobjoy J, Sarros S, Steidl S, Dürr M, Lacey DC, et al. Granulocyte-macrophage colony-stimulating factor is a key mediator in inflammatory and arthritic pain. Ann Rheum Dis. 2013;72:265–70.
Cook AD, Pobjoy J, Steidl S, Dürr M, Braine EL, Turner AL, et al. Granulocyte-macrophage colony-stimulating factor is a key mediator in experimental osteoarthritis pain and disease development. Arthritis Res Ther. 2012;14:R199.
Martinez-Moczygemba M, Huston DP. Biology of common beta receptor-signaling cytokines: IL-3, IL-5, and GM-CSF. J Allergy Clin Immunol. 2003;112:653–65. quiz 666.
Hercus TR, Thomas D, Guthridge MA, Ekert PG, King-Scott J, Parker MW, et al. The granulocyte-macrophage colony-stimulating factor receptor: linking its structure to cell signaling and its role in disease. Blood. 2009;114:1289–98.
Broughton SE, Nero TL, Dhagat U, Kan WL, Hercus TR, Tvorogov D, et al. The βc receptor family—structural insights and their functional implications. Cytokine. 2015;74:247–58.
Rosas M, Gordon S, Taylor PR. Characterisation of the expression and function of the GM-CSF receptor alpha-chain in mice. Eur J Immunol. 2007;37:2518–28.
Wang Y, Han G, Wang K, Liu G, Wang R, Xiao H, et al. Tumor-derived GM-CSF promotes inflammatory colon carcinogenesis via stimulating epithelial release of VEGF. Cancer Res. 2014;74:716–26.
Colotta F, Bussolino F, Polentarutti N, Guglielmetti A, Sironi M, Bocchietto E, et al. Differential expression of the common beta and specific alpha chains of the receptors for GM-CSF, IL-3, and IL-5 in endothelial cells. Exp Cell Res. 1993;206:311–7.
Prevost JM, Pelley JL, Zhu W, D'Egidio GE, Beaudry PP, Pihl C, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and inflammatory stimuli up-regulate secretion of the soluble GM-CSF receptor in human monocytes: evidence for ectodomain shedding of the cell surface GM-CSF receptor alpha subunit. J Immunol. 2002;169:5679–88.
Brown CB, Beaudry P, Laing TD, Shoemaker S, Kaushansky K. In vitro characterization of the human recombinant soluble granulocyte-macrophage colony-stimulating factor receptor. Blood. 1995;85:1488–95.
Shiomi A, Usui T. Pivotal roles of GM-CSF in autoimmunity and inflammation. Mediators Inflamm. 2015;2015:568543.
Hamilton JA. GM-CSF in inflammation and autoimmunity. Trends Immunol. 2002;23:403–8.
Quill H, Gaur A, Phipps RP. Prostaglandin E2-dependent induction of granulocyte-macrophage colony-stimulating factor secretion by cloned murine helper T cells. J Immunol. 1989;142:813–8.
Kleinewietfeld M, Hafler DA. The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin Immunol. 2013;25:305–12.
Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-β signalling. Nature. 2010;467:967–71.
Peters A, Lee Y, Kuchroo VK. The many faces of Th17 cells. Curr Opin Immunol. 2011;23:702–6.
Duhen T, Campbell DJ. IL-1β promotes the differentiation of polyfunctional human CCR6+ CXCR3+ Th1/17 cells that are specific for pathogenic and commensal microbes. J Immunol. 2014;193:120–9.
Maggi L, Santarlasci V, Capone M, Peired A, Frosali F, Crome SQ, et al. CD161 is a marker of all human IL-17-producing T-cell subsets and is induced by RORC. Eur J Immunol. 2010;40:2174–81.
Kara EE, McKenzie DR, Bastow CR, Gregor CE, Fenix KA, Ogunniyi AD, et al. CCR2 defines in vivo development and homing of IL-23-driven GM-CSF-producing Th17 cells. Nat Commun. 2015;6:8644.
O'Malley JT, Eri RD, Stritesky GL, Mathur AN, Chang HC, Hogenesch H, et al. STAT4 isoforms differentially regulate Th1 cytokine production and the severity of inflammatory bowel disease. J Immunol. 2008;181:5062–70.
Kolarz G, Scherak O, Popp W, Ritschka L, Thumb N, Wottawa A, et al. Bronchoalveolar lavage in rheumatoid arthritis. Br J Rheumatol. 1993;32:556–61.
Sheng W, Yang F, Zhou Y, Yang H, Low PY, Kemeny DM, et al. STAT5 programs a distinct subset of GM-CSF-producing T helper cells that is essential for autoimmune neuroinflammation. Cell Res. 2014;24:1387–402.
Noster R, Riedel R, Mashreghi MF, Radbruch H, Harms L, Haftmann C, et al. IL-17 and GM-CSF expression are antagonistically regulated by human T helper cells. Sci Transl Med. 2014;6:241ra280.
Cousins DJ, Lee TH, Staynov DZ. Cytokine coexpression during human Th1/Th2 cell differentiation: direct evidence for coordinated expression of Th2 cytokines. J Immunol. 2002;169:2498–506.
Nakamura Y, Christodoulopoulos P, Cameron L, Wright E, Lavigne F, Toda M, et al. Upregulation of the transcription factor GATA-3 in upper airway mucosa after in vivo and in vitro allergen challenge. J Allergy Clin Immunol. 2000;105:1146–52.
Cook AD, Braine EL, Campbell IK, Rich MJ, Hamilton JA. Blockade of collagen-induced arthritis post-onset by antibody to granulocyte-macrophage colony-stimulating factor (GM-CSF): requirement for GM-CSF in the effector phase of disease. Arthritis Res. 2001;3:293–8.
Shiomi A, Usui T, Ishikawa Y, Shimizu M, Murakami K, Mimori T. GM-CSF but not IL-17 is critical for the development of severe interstitial lung disease in SKG mice. J Immunol. 2014;193:849–59.
Campbell IK, Rich MJ, Bischof RJ, Dunn AR, Grail D, Hamilton JA. Protection from collagen-induced arthritis in granulocyte-macrophage colony-stimulating factor-deficient mice. J Immunol. 1998;161:3639–44.
Kitching AR, Ru Huang X, Turner AL, Tipping PG, Dunn AR, Holdsworth SR. The requirement for granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor in leukocyte-mediated immune glomerular injury. J Am Soc Nephrol. 2002;13:350–8.
Schön M, Denzer D, Kubitza RC, Ruzicka T, Schön MP. Critical role of neutrophils for the generation of psoriasiform skin lesions in flaky skin mice. J Invest Dermatol. 2000;114:976–83.
Sheng JR, Muthusamy T, Prabhakar BS, Meriggioli MN. GM-CSF-induced regulatory T cells selectively inhibit anti-acetylcholine receptor-specific immune responses in experimental myasthenia gravis. J Neuroimmunol. 2011;240–241:65–73.
Gaudreau S, Guindi C, Ménard M, Besin G, Dupuis G, Amrani A. Granulocyte-macrophage colony-stimulating factor prevents diabetes development in NOD mice by inducing tolerogenic dendritic cells that sustain the suppressive function of CD4+CD25+ regulatory T cells. J Immunol. 2007;179:3638–47.
Sainathan SK, Hanna EM, Gong Q, Bishnupuri KS, Luo Q, Colonna M, et al. Granulocyte macrophage colony-stimulating factor ameliorates DSS-induced experimental colitis. Inflamm Bowel Dis. 2008;14:88–99.
Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–41.
Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol. 2009;9:393–407.
Perrella O, Carrieri PB, De Mercato R, Buscaino GA. Markers of activated T lymphocytes and T cell receptor gamma/delta+ in patients with multiple sclerosis. Eur Neurol. 1993;33:152–5.
McQualter JL, Darwiche R, Ewing C, Onuki M, Kay TW, Hamilton JA, et al. Granulocyte macrophage colony-stimulating factor: a new putative therapeutic target in multiple sclerosis. J Exp Med. 2001;194:873–82.
Ponomarev ED, Shriver LP, Maresz K, Pedras-Vasconcelos J, Verthelyi D, Dittel BN. GM-CSF production by autoreactive T cells is required for the activation of microglial cells and the onset of experimental autoimmune encephalomyelitis. J Immunol. 2007;178:39–48.
González H, Pacheco R. T-cell-mediated regulation of neuroinflammation involved in neurodegenerative diseases. J Neuroinflammation. 2014;11:201.
Parajuli B, Sonobe Y, Kawanokuchi J, Doi Y, Noda M, Takeuchi H, et al. GM-CSF increases LPS-induced production of proinflammatory mediators via upregulation of TLR4 and CD14 in murine microglia. J Neuroinflammation. 2012;9:268.
King IL, Dickendesher TL, Segal BM. Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood. 2009;113:3190–7.
Aubé B, Lévesque SA, Paré A, Chamma É, Kébir H, Gorina R, et al. Neutrophils mediate blood-spinal cord barrier disruption in demyelinating neuroinflammatory diseases. J Immunol. 2014;193:2438–54.
Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix CC. CNS immune privilege: hiding in plain sight. Immunol Rev. 2006;213:48–65.
Hirota K, Hashimoto M, Yoshitomi H, Tanaka S, Nomura T, Yamaguchi T, et al. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J Exp Med. 2007;204:41–7.
Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–7.
Kellner H. Targeting interleukin-17 in patients with active rheumatoid arthritis: rationale and clinical potential. Ther Adv Musculoskelet Dis. 2013;5:141–52.
Xu WD, Firestein GS, Taetle R, Kaushansky K, Zvaifler NJ. Cytokines in chronic inflammatory arthritis. II. Granulocyte-macrophage colony-stimulating factor in rheumatoid synovial effusions. J Clin Invest. 1989;83:876–82.
Fiehn C, Wermann M, Pezzutto A, Hüfner M, Heilig B. Plasma GM-CSF concentrations in rheumatoid arthritis, systemic lupus erythematosus and spondyloarthropathy. Z Rheumatol. 1992;51:121–6.
Hazenberg BP, Van Leeuwen MA, Van Rijswijk MH, Stern AC, Vellenga E. Correction of granulocytopenia in Felty’s syndrome by granulocyte-macrophage colony-stimulating factor. Simultaneous induction of interleukin-6 release and flare-up of the arthritis. Blood. 1989;74:2769–70.
Kotake S, Higaki M, Sato K, Himeno S, Morita H, Kim KJ, et al. Detection of myeloid precursors (granulocyte/macrophage colony forming units) in the bone marrow adjacent to rheumatoid arthritis joints. J Rheumatol. 1992;19:1511–6.
Menaa C, Kurihara N, Roodman GD. CFU-GM-derived cells form osteoclasts at a very high efficiency. Biochem Biophys Res Commun. 2000;267:943–6.
Piper C, Pesenacker AM, Bending D, Thirugnanabalan B, Varsani H, Wedderburn LR, et al. T cell expression of granulocyte-macrophage colony-stimulating factor in juvenile arthritis is contingent upon Th17 plasticity. Arthritis Rheumatol. 2014;66:1955–60.
Campbell IK, Bendele A, Smith DA, Hamilton JA. Granulocyte-macrophage colony stimulating factor exacerbates collagen induced arthritis in mice. Ann Rheum Dis. 1997;56:364–8.
Hashimoto M, Hirota K, Yoshitomi H, Maeda S, Teradaira S, Akizuki S, et al. Complement drives Th17 cell differentiation and triggers autoimmune arthritis. J Exp Med. 2010;207:1135–43.
Keith RC, Powers JL, Redente EF, Sergew A, Martin RJ, Gizinski A, et al. A novel model of rheumatoid arthritis-associated interstitial lung disease in SKG mice. Exp Lung Res. 2012;38:55–66.
Johnson GR, Gonda TJ, Metcalf D, Hariharan IK, Cory S. A lethal myeloproliferative syndrome in mice transplanted with bone marrow cells infected with a retrovirus expressing granulocyte-macrophage colony stimulating factor. EMBO J. 1989;8:441–8.
Xing Z, Ohkawara Y, Jordana M, Graham F, Gauldie J. Transfer of granulocyte-macrophage colony-stimulating factor gene to rat lung induces eosinophilia, monocytosis, and fibrotic reactions. J Clin Invest. 1996;97:1102–10.
White ES, Lazar MH, Thannickal VJ. Pathogenetic mechanisms in usual interstitial pneumonia/idiopathic pulmonary fibrosis. J Pathol. 2003;201:343–54.
Garcia JG, Parhami N, Killam D, Garcia PL, Keogh BA. Bronchoalveolar lavage fluid evaluation in rheumatoid arthritis. Am Rev Respir Dis. 1986;133:450–4.
Garcia JG, James HL, Zinkgraf S, Perlman MB, Keogh BA. Lower respiratory tract abnormalities in rheumatoid interstitial lung disease. Potential role of neutrophils in lung injury. Am Rev Respir Dis. 1987;136:811–7.
Xing Z, Braciak T, Ohkawara Y, Sallenave JM, Foley R, Sime PJ, et al. Gene transfer for cytokine functional studies in the lung: the multifunctional role of GM-CSF in pulmonary inflammation. J Leukoc Biol. 1996;59:481–8.
Taniguchi H, Katoh S, Kadota J, Matsubara Y, Fukushima K, Mukae H, et al. Interleukin 5 and granulocyte-macrophage colony-stimulating factor levels in bronchoalveolar lavage fluid in interstitial lung disease. Eur Respir J. 2000;16:959–64.
Walker C, Bauer W, Braun RK, Menz G, Braun P, Schwarz F, et al. Activated T cells and cytokines in bronchoalveolar lavages from patients with various lung diseases associated with eosinophilia. Am J Respir Crit Care Med. 1994;150:1038–48.
Izumi K, Hashizume M, Yoshimoto K, Kaneko Y, Yasuoka H, Suzuki K, Yamaoka K, Takeuchi Y. Serum GM-CSF levels are significantly associated with interstitial pneumonia in biologic-native patients with rheumatoid arthritis: a single-center prospective cohort study (Keio First-bio cohort study). In EULAR 2015. Roma: 2015
Bernasconi E, Favre L, Maillard MH, Bachmann D, Pythoud C, Bouzourene H, et al. Granulocyte-macrophage colony-stimulating factor elicits bone marrow-derived cells that promote efficient colonic mucosal healing. Inflamm Bowel Dis. 2010;16:428–41.
Däbritz J. Granulocyte macrophage colony-stimulating factor and the intestinal innate immune cell homeostasis in Crohn’s disease. Am J Physiol Gastrointest Liver Physiol. 2014;306:G455–465.
Gennari R, Alexander JW, Gianotti L, Eaves-Pyles T, Hartmann S. Granulocyte macrophage colony-stimulating factor improves survival in two models of gut-derived sepsis by improving gut barrier function and modulating bacterial clearance. Ann Surg. 1994;220:68–76.
Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–17.
Noguchi M, Hiwatashi N, Liu ZX, Toyota T. Increased secretion of granulocyte-macrophage colony-stimulating factor in mucosal lesions of inflammatory bowel disease. Digestion. 2001;63 Suppl 1:32–6.
Ina K, Kusugami K, Hosokawa T, Imada A, Shimizu T, Yamaguchi T, et al. Increased mucosal production of granulocyte colony-stimulating factor is related to a delay in neutrophil apoptosis in inflammatory bowel disease. J Gastroenterol Hepatol. 1999;14:46–53.
Dieleman LA, Ridwan BU, Tennyson GS, Beagley KW, Bucy RP, Elson CO. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology. 1994;107:1643–52.
Han X, Uchida K, Jurickova I, Koch D, Willson T, Samson C, et al. Granulocyte-macrophage colony-stimulating factor autoantibodies in murine ileitis and progressive ileal Crohn’s disease. Gastroenterology. 2009;136:1261–71. e1261-1263.
Xu Y, Hunt NH, Bao S. The role of granulocyte macrophage-colony-stimulating factor in acute intestinal inflammation. Cell Res. 2008;18:1220–9.
Egea L, Hirata Y, Kagnoff MF. GM-CSF: a role in immune and inflammatory reactions in the intestine. Expert Rev Gastroenterol Hepatol. 2010;4:723–31.
Khajah M, Millen B, Cara DC, Waterhouse C, McCafferty DM. Granulocyte-macrophage colony-stimulating factor (GM-CSF): a chemoattractive agent for murine leukocytes in vivo. J Leukoc Biol. 2011;89:945–53.
Griseri T, McKenzie BS, Schiering C, Powrie F. Dysregulated hematopoietic stem and progenitor cell activity promotes interleukin-23-driven chronic intestinal inflammation. Immunity. 2012;37:1116–29.
Biondo M, Nasa Z, Marshall A, Toh BH, Alderuccio F. Local transgenic expression of granulocyte macrophage-colony stimulating factor initiates autoimmunity. J Immunol. 2001;166:2090–9.
Dieckgraefe BK, Korzenik JR. Treatment of active Crohn’s disease with recombinant human granulocyte-macrophage colony-stimulating factor. Lancet. 2002;360:1478–80.
Korzenik JR, Dieckgraefe BK, Valentine JF, Hausman DF, Gilbert MJ. Group SiCsDS: sargramostim for active Crohn’s disease. N Engl J Med. 2005;352:2193–201.
Valentine JF, Fedorak RN, Feagan B, Fredlund P, Schmitt R, Ni P, et al. Steroid-sparing properties of sargramostim in patients with corticosteroid-dependent Crohn’s disease: a randomised, double-blind, placebo-controlled, phase 2 study. Gut. 2009;58:1354–62.
Roth L, MacDonald JK, McDonald JW, Chande N. Sargramostim (GM-CSF) for induction of remission in Crohn’s disease: a cochrane inflammatory bowel disease and functional bowel disorders systematic review of randomized trials. Inflamm Bowel Dis. 2012;18:1333–9.
Constantinescu CS, Asher A, Fryze W, Kozubski W, Wagner F, Aram J, et al. Randomized phase 1b trial of MOR103, a human antibody to GM-CSF, in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2015;2:e117.
Behrens F, Tak PP, Ostergaard M, Stoilov R, Wiland P, Huizinga TW, Berenfus VY, Vladeva S, Rech J, Rubbert-Roth A, et al. MOR103, a human monoclonal antibody to granulocyte-macrophage colony-stimulating factor, in the treatment of patients with moderate rheumatoid arthritis: results of a phase Ib/IIa randomised, double-blind, placebo-controlled, dose-escalation trial. Ann Rheum Dis. 2015;74(6):1058–64. doi:10.1136/annrheumdis-2013-204816. Epub 2014 Feb 17.
Burmester GR, Weinblatt ME, McInnes IB, Porter D, Barbarash O, Vatutin M, Szombati I, Esfandiari E, Sleeman MA, Kane CD, et al. Efficacy and safety of mavrilimumab in subjects with rheumatoid arthritis. Ann Rheum Dis. 2013;72(9):1445–52. doi:10.1136/annrheumdis-2012-202450. Epub 2012 Dec 12.
Uchida K, Nakata K, Suzuki T, Luisetti M, Watanabe M, Koch DE, et al. Granulocyte/macrophage-colony-stimulating factor autoantibodies and myeloid cell immune functions in healthy subjects. Blood. 2009;113:2547–56.
Sakagami T, Beck D, Uchida K, Suzuki T, Carey BC, Nakata K, et al. Patient-derived granulocyte/macrophage colony-stimulating factor autoantibodies reproduce pulmonary alveolar proteinosis in nonhuman primates. Am J Respir Crit Care Med. 2010;182:49–61.
Hirata Y, Egea L, Dann SM, Eckmann L, Kagnoff MF. GM-CSF-facilitated dendritic cell recruitment and survival govern the intestinal mucosal response to a mouse enteric bacterial pathogen. Cell Host Microbe. 2010;7:151–63.
Burmester GR, Feist E, Sleeman MA, Wang B, White B, Magrini F. Mavrilimumab, a human monoclonal antibody targeting GM-CSF receptor-α, in subjects with rheumatoid arthritis: a randomised, double-blind, placebo-controlled, phase I, first-in-human study. Ann Rheum Dis. 2011;70:1542–9.
Burmester G, McInnes IB, Kremer JM, Miranda P, Korkosz M, Vencovsky J, et al. Efficacy and safety/tolerability of mavrilimumab, a human GM-CSFRá monoclonal antibody in patients with rheumatoid arthritis. Boston: American College of Rheumatology; 2014.
Kremer JM, Burmester G, Weinblatt M, Williams A, Karlsson N, Godwood A, et al. Analysis of patient-reported outcomes during treatment with mavrilimumab, a human monoclonal antibody targeting GM-CSFRá, in the randomized Phase 2b Earth Explorer 1 Study. Boston: American College of Rheumatology; 2014.
McInnes IB, Burmester G, Kremer JM, Miranda P, Korkosz M, Vencovsky J, et al. Rapid onset of clinical benefit is associated with a reduction in validated biomarkers of disease in patients with rheumatoid arthritis treated with mavrilimumab, a human monoclonal antibody targeting GM-CSFRá. Boston: American College of Rheumatology; 2014.
A study of mavrilimumab in subjects with moderate-to-severe rheumatoid arthritis. https://clinicaltrials.gov/ct2/show/NCT01706926.
A long term safety study of mavrilimumab in adult subjects with rheumatoid arthritis. https://clinicaltrials.gov/ct2/show/study/NCT01712399.
Burmester G, McInnes I, Kremer J, Miranda P, Vencovský J, Godwood A, et al. Long-term safety and efficacy of mavrilimumab, a fully human granulocyte-macrophage colony-stimulating factor receptor-α (GM–CSFR-α) monoclonal antibody, in patients with rheumatoid arthritis (RA). San Francisco: American College of Rheumatology; 2015.
A study of mavrilimumab versus anti tumor necrosis factor in subjects with rheumatoid arthritis. https://clinicaltrials.gov/ct2/show/study/NCT01715896
A trial to evaluate the safety and tolerability of namilumab (MT203) in patients with mild to moderate rheumatoid arthritis (PRIORA). https://clinicaltrials.gov/ct2/show/study/NCT01317797.
Dose finding study of namilumab in combination with methotrexate in participants with moderate to severe rheumatoid arthritis (RA). https://clinicaltrials.gov/ct2/show/NCT02379091.
Namilumab vs adalimumab in participants with moderate to severe early rheumatoid arthritis inadequately responding to methotrexate (TELLUS). https://clinicaltrials.gov/ct2/show/NCT02393378.
Efficacy and safety of namilumab (MT203) for plaque psoriasis. https://clinicaltrials.gov/ct2/show/NCT02129777.
Study of KB003 in biologics-inadequate rheumatoid arthritis. https://clinicaltrials.gov/ct2/show/results/NCT00995449.
Safety and tolerability of MORAb-022 in healthy and rheumatoid arthritis subjects. https://clinicaltrials.gov/ct2/show/NCT01357759.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AS drafted the manuscript. TU helped to draft the manuscript. TM gave the final approval of the version to be submitted and any revised version. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Shiomi, A., Usui, T. & Mimori, T. GM-CSF as a therapeutic target in autoimmune diseases. Inflamm Regener 36, 8 (2016). https://doi.org/10.1186/s41232-016-0014-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41232-016-0014-5