Abstract
Background
Immune Checkpoint Inhibitor (ICI) immunotherapy is most effective in immune effector cell infiltrated ‘hot’ tumor lesions, such as occurs in deficient mismatch repair, microsatellite instability high (dMMR/MSI-H) colorectal cancer (CRC). However, most all metastatic CRC tumors are mismatch repair proficient/microsatellite stable (pMMR/MSS) ‘cold’ lesions, without significant immune cell infiltration, and are unresponsive to ICI. AlloStim®, is an experimental, allogeneic immunomodulatory cell therapy designed to convert ‘cold’ metastatic tumor lesions to ‘hot’ inflamed lesions. After AlloStim® immunotherapy, this cold to hot inflammatory mechanism can make it difficult to distinguish between pseudoprogression and actual progression on restaging CT scans, as inflamed metastatic lesions can appear larger and occult disease can appear as new small lesions.
Methods
To explore whether radiological progression after AlloStim® immunotherapy is due to immune-flare or disease progression, we administered a short course of a combination ICI therapy to a pMMR/MSS chemotherapy-refractory metastatic colorectal cancer patient enrolled in the StimVax Phase IIb clinical study that presented with radiological progression after AlloStim® immunotherapy. Our rationale was that an accelerated response to ICI should occur if the lesions were inflamed, while if the enlarged lesions were due to disease progression there would not be a response.
Results
Here we report a rapid, significant reduction in tumor burden in response to ICI administration in an AlloStim® primed pMMR/MSS mCRC patient with retroperitoneal and lung metastases.
Conclusion
This rare objective response to ICIs in a pMMR/MSS mCRC patient supports further evaluation of the combination of AlloStim® with ICI immunotherapy in MSS mCRC and other cold or ICI refractory tumors.
Trial registration
National Library of Medicine (NLM) at the National Institutes of Health (NIH). Registered 22 June 2020, https://clinicaltrials.gov/study/NCT04444622.
Similar content being viewed by others
Background
Immune checkpoint inhibitor (ICI)-based regimens have not yet shown meaningful positive outcomes in proficient DNA mismatch repair/microsatellite stable (pMMR/MSS) metastatic colorectal cancers (mCRC). Here we report a rare objective response in a pMMR/MSS heavily pre-treated metastatic colorectal cancer (mCRC) patient subsequent to a short course of an immune checkpoint inhibitor (ICI) combination after first being primed with an experimental immunomodulatory cell therapy drug, AlloStim®, designed to convert immunologically ‘cold’ tumors to ‘hot’ tumors, and a short, low dose course of regorafenib.
The FDA has approved ICI drugs targeting CTLA-4, PD-1, PD-L1 and LAG-3 checkpoint molecules for a variety of solid tumor indications, including melanoma, renal, bladder, lung, gastric, gastroesophageal junction, hepatocellular carcinoma and head and neck cancers. However, ICIs have demonstrated only limited efficacy in mCRC.
An anti-CTLA4 ICI, pembrolizumab, was approved in first line mCRC [1] and also approved in combination with the anti-PD-1 ICI, nivolumab, for a subset of mCRC patients that have deficient DNA mismatch repair/microsatellite instability-high (dMMR/MSI-H) status [2,3,4,5,6,7,8]. However, this dMMR/MSI-H subset constitutes only ~ 5% of mCRC patients [9], while the remaining ~ 95% that present with pMMR/MSS status do not respond to ICI [6, 8, 10,11,12,13].
Most dMMR/MSI-H status tumors are considered immunologically ‘hot’ tumors, while pMMR/MSS status tumors are considered to be ‘cold’ [14]. ICI have demonstrated greater efficacy in hot tumors, characterized by an inflamed phenotype, including a high level of infiltrating T-cells and NK cells, an interferon-γ (IFN-γ) signature and upregulated PD-L1 expression, while cold tumors have an absence of tumor-infiltrating lymphocytes [15].
While dMMR/MSI-H mCRC patients are more responsive to ICI therapy, approximately 50% are refractory [16,17,18]. Resistance to ICI responsiveness, regardless of MMR/MSI status, is correlated with tumor mutational burden (TMB) [19]. Since somatic mutations can encode immunogenic neoantigens, high TMB is believed to be more likely to prime for infiltrating tumor-specific effector immune cells. Consistent with this, the dMMR/MSI-H patients that present with lower TMB values have been shown to be the non-responders, whereas patients with the highest TMB values tend to obtain benefit from ICI [16], particularly with anti-CTLA-4/PD-1 combination ICI immunotherapy [20].
Present strategies for increasing the effectiveness of ICI immunotherapy in pMMR/MSS mCRC cold tumors include evaluating combinations with other therapeutic methods, such as chemotherapy, targeted therapy, and radiotherapy [21]. In addition, strategies to convert immunologically cold tumors to hot tumors are thought to be a fruitful line of investigation for increasing ICI efficacy in ICI refractory tumors [22, 23].
AlloStim® is an experimental cellular immunotherapy drug designed to convert immunologically cold tumors to hot tumors by mirroring the graft vs. tumor (GVT) mechanism of allogeneic stem cell transplant procedures to create a host vs tumor (HVT) effect without graft vs. host disease (GVHD) toxicity [24]. AlloStim® is currently being evaluated as a monotherapy in the STIMVAX Phase IIB open label clinical trial in third-line chemotherapy-refractory pMMR/MSS mCRC (NCT04444622). The primary end-point in this study is overall survival (OS) and an exploratory end-point is objective tumor response by RECIST 1.1.
AlloStim® is a living, allogeneic (“off-the-shelf”), non-genetically manipulated, activated Th1-like immune cell therapy derived from CD4 + T-cell precursors isolated from the blood of healthy donors. The STIMVAX protocol provides for three monthly cycles of weekly AlloStim® administration (intradermal and intravenous) designed to increase circulating memory Th1/Th2 ratio [25], activate circulating memory T cells and NK cells, which in turn causes their extravasation to tumor sites [24]. The anti-tumor effects are correlated with the establishment of an IFN-γ dominated microenvironment [26]. The systemic tumor infiltration mechanism serves to convert immunologically cold tumors to hot tumors. The modulation of the tumor microenvironment (TME) can also counter-regulate tumor-mediated immune suppression [27].
AlloStim®-mediated intratumoral type I cytokine production by infiltrating activated T-cells and NK cells, including IL-12 and IFN-γ, is believed to: upregulate MHC-I on tumor cells making them susceptible to CD8 + T-cell recognition; cause maturation of dendritic cells to DC1 (IL-12 + CD80/86 positive, MHC I and MHC II positive); convert M2 macrophages to M1 [28]; and, release neoantigens into the TME [29, 30]. The release of neoantigens into an inflammatory TME creates the conditions for in-situ vaccination [31] where immature dendritic cells mature to type I dendritic cells (DC1), process the released chaperoned neoantigens, migrate to the draining lymph nodes, resulting in a patient-specific anti-tumor adaptive immune response [32].
In previous clinical studies, the inflammatory mechanism of AlloStim® almost always caused post-treatment CT scan images to be read as progressive disease (PD), with systemic increases in size of existing tumor lesions and often the appearance of new small lesions (especially in lungs). However, this PD determination did not always correlate with the clinical status of the patient or with overall survival (OS). As the systemic increase in target lesion size could be due to peritumoral inflammation that occurs when tumor lesions convert from cold to hot and new small lesions could be the result of inflammation of occult disease, it is difficult to distinguish tumor progression from pseudoprogression using CT scan imaging after experimental AlloStim® experimental treatment.
Pseudoprogression after immunotherapy has been observed in patients with various tumor types and is thought to be due to transient immune cell infiltration into the tumor [33]. The phenomenon of pseudoprogression has led to modification of the RECIST 1.1 evaluation criteria [34]. The understanding that tumor growth by RECIST does not necessarily translate to disease progression in patients treated with immunotherapy has also led to the development of immune-related response criteria (irRC) to better surveil these patients [35, 36].
However, it is still considered challenging to distinguish radiological progression from pseudoprogression and, consequently, to define the best management for these patients. In additional, a new category of “hyper-progression” and dissociated atypical responses have also been described after immunotherapy [37]. These issues have resulted in some subjects being prematurely removed from immunotherapy clinical trials [38].
In the CheckMate 142 clinical trial, nivolumab (3 mg/kg) plus low-dose ipilimumab (1 mg/kg) provided durable clinical benefit, and a manageable safety profile in patients with previously treated dMMR/MSI-H metastatic CRC [39]. Due to the improved safety profile of the lower dose ipilimumab in this combination, we decided to test this regimen in a chemotherapy-refractory mCRC pMMR/MSS patient presenting with radiological progression after 3 cycles of AlloStim® experimental immunotherapy.
We hypothesized that if the radiological progression was due to inflammation within the tumor lesions (hot tumors), a short course of ICI immunotherapy should result in a rapid reduction in tumor burden, due to release of suppression of resident infiltrating effector immune cells. On the other hand, if the enlarged and increased number of lesions were due to true progression and no infiltrating effector immune cells were present, either no response or further progression would be expected to be observed.
Case presentation
A 69 yo Caucasian male presented in March 2011 with blood in the stool. The patient’s medical and social history was significant for type 2 diabetes, hypertension, hypercholesterolemia, atrial fibrillation (s/p ablation) and was a former 2 pack/day smoker. Upon workup, was found to have a rectal mass and subsequently underwent low anterior resection (LAR). 2 of 24 nodes were found to be positive for adenocarcinoma disease with initial staging of pT2N1b. The patient was treated with adjuvant FOLFOX and achieved a complete response (CR). In August 2014, disease recurred and he underwent transanal resection plus radiation therapy (XRT). In April 2016, a right inguinal node was identified as metastatic adenocarcinoma and he received additional XRT. In January 2022, he presented with enlarged retroperitoneal nodes and was treated with 22 cycles of FOLFIRI plus Avastin from January 2022 to November 2022. In January 2023, was treated again with FOLFOX, but developed a reaction to oxaliplatin.
CT scan on April 26, 2023 showed appearance of innumerable bilateral pleural parenchymal lung nodules significantly increased in size and number from prior examination on March 8, 2023 and significant worsening of mediastinal and hilar lymphadenopathy consistent with progressive metastatic disease. No suspicious liver lesions were found and stable non-specific retroperitoneal adenopathy was also noted. Target lesions were identified in the lungs. The retroperitoneal disease was too small (< 15 mm) to be included in the RECIST 1.1 evaluation.
Patient consented on April 21, 2023 to received AlloStim® experimental immunotherapy as part of the STIMVAX Phase IIB clinical trial (NCT04444622). Eligible patients had histologically confirmed pMMR/MSS adenocarcinoma of the colon or rectum; received all available standard systemic therapies (fluoropyrimidines, oxaliplatin, irinotecan, and bevacizumab; cetuximab or panitumumab if RAS wild-type tumors); were aged 18 years or older; had adequate organ function; Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and measurable disease. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines after approval by a central institutional review board (IRB) and the ethics board at each institution where applicable.
Results
The dosing and procedure schedule is shown in Table 1 and longitudinal changes in lung target lesions from CT scans are shown in Fig. 1. Three 28-day cycles of weekly AlloStim® immunotherapy were administered per protocol from May 9, 2023 to September 26, 2023. The CT scan comparison from baseline (April 26, 2023) to completion of the AlloStim® three cycles (October 10, 2023) demonstrated progressive disease (PD) by RECIST 1.1 evaluating target lesions in the lungs and retroperitoneum (sum of diameters of target lesions = + 73%) with increased non-target retroperitoneal adenopathy and innumerable enlarging and new thoracic nodules.
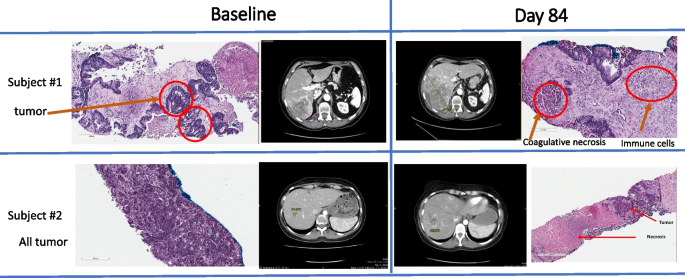
Matched CT scans and biopsies of liver target lesions in two MSS mCRC subjects at baseline and at day 84 after 3 AlloStim® cycles. Subject #1 shows extensive tumor (red circle) on the periphery of areas of fibrosis without immune cell infiltration at baseline. The corresponding CT scan shows the biopsied target tumor in liver After 3 cycles of AlloStim®, the re-staging CT scan on day 84 shows progressive disease. However, the corresponding biopsies show areas of coagulative necrosis and immune cell infiltration. Subject #2 has almost completely solid tumor at baseline. The re-staging CT scan indicates progressive disease, however note the extensive peri-tumoral inflammation. The corresponding biopsy indicates large area of tumor necrosis and tumor admixed with immune cells
From October 11, 2023 to October 23, 2023, a short, reduced dose of regorafenib was administered followed by a short course of nivolumab (240 mg) and low dose ipilimumab (1 mg/kg) administered between October 23, 2023 and December 4, 2023. No treatment was administered from December 12, 2023 and February 8, 2024 during which time a tapering dose of oral prednisone beginning at 60 mg/day was administered for treatment of colitis (adverse effect from the combination ICI immunotherapy), a restaging CT scan was then obtained on February 8, 2024.
The February 8, 2024 scan demonstrated a partial response (PR) by RECIST 1.1 criteria in comparison to the post-AlloStim® scan on October 10, 2023 (change in sum of diameters of target lesions = -48%) with the previously observed innumerable non-target thoracic lesions and retroperitoneal adenopathy uniformly decreased in size and number. Comparison to the April 26, 2023 baseline scan was read as stable disease (SD) with a 10% decrease in sum of diameters of target lesions (see Fig. 2).
Longitudinal changes in two lung target lesions (orange arrows). The slices are adjusted to show the view with the measurement in the longest tumor diameter. Red circles indicate presence or absence of non-target lesions in the selected slices. An increase in size of non-target existing lesions and appearance of new non-target lesions seen on post-AlloStim October 10, 2023 compared to April 26, 2023 baseline. Elimination or reduction in size of the non-target lesions seen in the post-ICI February 8, 2024 scan. According to RECIST 1.1, the October 10, 2023 scan compared to April 26, 2023 is scored as PD. The February 8, 2024 scan compared to October 10, 2023 is scored as PR. The February 8, 2024 compared to April 26, 2023 is scored as SD.
Serum levels of IL-12 levels were negligible prior to AlloStim® administration. After AlloStim® dosing, IL-12 became detectable after the first cycle and remained detectible over the 3 cycles of AlloStim® administration. Soluble heat shock protein (HSP)-70 was also negligible at baseline but was elevated throughout the experimental immunotherapy dosing (see Fig. 3).
IL-12 and HSP70 serum levels during allostim administration. Whole blood samples were collected longitudinally from subjects in SST Tiger Top tubes. The tubes were spun at 3000 rpm and shipped overnight at 2-80C to the central lab facility where the serum was aseptically transferred to cryotubes and stored at -800C until analysis. For analysis, samples were diluted 1:2 and plated in triplicate on ELISA plates (R&D Systems) and incubated for 2-3h at RT. The plates were read on a Cytation 7 plate reader (Agilent BioTek) at 650nm absorbance. A standard curve was generated using known samples. Quantitative levels were determined by comparing absorbance values to the standard curve. The bar graph shows the mean +/- SE at each sample timepoint
Discussion
Here we present a rare case of rapid tumor debulking response subsequent to a short course of combination ICI immunotherapy in a heavily pre-treated pMMR/MSS mCRC patient presenting with radiological progression after third-line experimental AlloStim® immunotherapy and a short course of low dose regorafenib. Here we consider the question whether the ICI immunotherapy alone or in combination with previous AlloStim®, or in combination with previous regorafenib alone, or with prior AlloStim® and regorafenib together was most likely responsible for eliciting this rare objective response in a cold tumor indication.
It seems unlikely that the combination ICI immunotherapy could be solely responsible for the observed response. ICI-based regimens both as monotherapy [40, 41] or as combination therapies [42] have not previously shown any meaningful positive outcomes in pMMR/MSS colorectal cancers.
For example, an initial phase II study assessed the efficacy of tremelimumab, a monoclonal antibody against CTLA4, in patients with treatment-refractory mCRC, which resulted in no improvement post-treatment [43]. Furthermore, two phase I studies of anti-PD-1 [44] and anti-PD-L1 [45] in previously-treated mCRC patients produced no responses. ICI regimens also failed as maintenance therapy after first line therapy in the MODUL study [46].
In general, ICI immunotherapy combining CTLA-4 and PD-L1 inhibitors have also shown very limited clinical benefit in patients with non-selected mCRC. A rare partial response (PR) (1/119) was reported in a randomized phase 2 clinical trial which evaluated the efficacy of combination durvalumab (anti-PD-L1) and tremelimumab (anti-CTLA-4) in patients with advanced refractory mCRC. In this study, 119 patients were assigned to the treatment group and 61 patients were assigned to best supportive care (BSC) alone. Patients in the treatment group received a median of 12 weeks of durvalumab and 12 weeks of tremelimumab [47], while in the present case only 5 weeks of ICI combination therapy was administered.
The phase II KEYNOTE-016 trial was performed to evaluate the clinical efficacy of single agent pembrolizumab in patients with pMMR/MSS mCRC, dMMR/MSI-H mCRC and or dMMR/MSI-H non-CRC. No responses were noted in 18 patients in the pMMR/MSS mCRC group [48]. In a clinical study which included 59 pMMR/MSS mCRC patients treated with ICI beyond radiological progression by RECIST 1.1, no patient demonstrated subsequent radiographical tumor shrinkage at a median of 42 days [49].
It has been reported that a small subset (~ 2%) of patients with pMMR/MSS colorectal cancer with a mutation in POLE and POLD1 enzymes and those without liver metastases have a higher chance of a response to ICI immunotherapy [50]. While the patient in the present case did not have POLE or POLD1 mutations, no liver metastases were present. Therefore, it is possible this patient was more susceptible to ICI immunotherapy, but seems unlikely that the short course of combination ICI alone was solely responsible for the extensive tumor debulking observed.
ICI strategies in combination with other drugs or procedures are under investigation, including evaluations of ICI in combinations with chemotherapy, radiotherapy, vascular endothelial growth factor (VEGF)/VEGF receptor (VEGFR) inhibitors, mitogen-activated protein kinase (MEK) inhibitors, and signal transducer and activation of transcription 3 (STAT3) inhibitors [51]. However, these combination approaches have yet to demonstrate any significant anti-tumor activity in the clinical setting [12, 52, 53].
Could the short course of regorafenib alone or in combination with ICI immunotherapy be responsible for the objective tumor response observed in this case?
Regorafenib is approved for third-line mCRC based on the results of the CORRECT trial which demonstrated only a 1.4 month increase in the median survival compared to a placebo control (6.4 months vs. 5.0 months) [54]. In the Phase II TEXCAN trial, no objective responses were reported in 35 mCRC patients after 2 months of treatment with regorafenib according to RECIST 1.1, Choi, and modified Choi [55]. Therefore, it seems unlikely that the prior short course of low dose regorafenib alone could be responsible for the rare objective response reported here.
Regorafenib is a multi-kinase inhibitor that targets several receptor tyrosine kinases involved in angiogenesis and metastases (VEGFR1, VEGFR2, VEGFR3, FGFR1, FGFR2, TIE2, PDGFRs), oncogenesis (KIT, RET, RAF1), and tumor immunity (CSF1R). While regorafenib does not directly convert cold tumors to hot tumors, regorafenib is believed to possibly contribute to shifting the tumor microenvironment toward a more immune-responsive state. This constellation of mechanisms suggests that regorafenib could potentially be a combination partner for ICIs [56].
There are mixed results on the combination of regorafenib with ICI in clinical trials. Regorafenib in combination with PD‐1 antibody as a third‐line mCRC therapy has been evaluated in several studies. For example, 24 patients with MSS mCRC were included in the REGONIVO study. In this study, regorafenib was administered at 80–160 mg once daily for 21 days on and 7 days off together with nivolumab at 3 mg/kg every 2 weeks. A 33.3% objective response rate was reported with this regimen [57]. However, this promising activity has not been observed in other studies.
In a single site study, 18 mCRC patients treated with a combination of regorafenib and nivolumab, no objective responses were observed. In this study, 13 patients (69%) had progressive disease, and the median progression-free survival (PFS) was only 2 months. Four out of five patients in this study evaluated with stable disease (SD) occurred in patients without liver metastases, whereas a short disease stabilization was seen in 1 of 14 patients with history of liver metastases [58].
In another study in MSS mCRC patients, a combination of regorafenib and toripalimab, an anti-PD-1 ICI yielded an objective response rate of 15.2% (5 of 33 patients) with all (3 of 3) with lung-only metastasis responding [59]. In a retrospective study that involved 14 Chinese medical centers, a partial response rate of 5% (4 of 84 patients) was reported in MSS mCRC patients administered regorafenib combined with ICIs [60].
In a phase 2 study in patients from the USA with pMMR/MSS mCRC, regorafenib plus nivolumab yielded an objective response rate of 7%, with all responses observed in patients without liver metastases [61]. In this study, regorafenib was administered at 80 mg/day on a 3 weeks on/1 week off schedule and was increased to 120 mg/day if the 80 mg/day was well tolerated. Nivolumab was administered at 480 mg every 4 weeks.
Based on these data, we cannot rule out the possibility that the regorafenib pre-treatment may have primed for responsiveness to the ICI immunotherapy in this pMMR/MSS mCRC patient that presented without liver metastases.
However, in this case, the doses and frequencies of both regorafenib and of the combination ICI immunotherapy that were actually administered were significantly less that the doses administered in clinical trials where objective responses were observed.
In addition, in the present case, corticosteroids (CS) were administered 6 weeks after start of ICI administration. In a retrospective single institution study, patients were evaluated in two cohorts based on timing of initiation of CS after initiation of ICI immunotherapy (≥ 2 months vs < 2 months). The administration of CS < 2 months after initiation of ICI immunotherapy was found to significantly hinder ICI efficacy [62].
Since regorafenib does not directly convert cold tumors to hot tumors, which is necessary for priming ICI responsiveness, and the doses and frequencies of both regorafenib and the ICI immunotherapy used in the present case were at sub-optimal therapeutic levels, combined with the early use of CS, we believe, while possible, it is unlikely that the regorafenib priming was responsible for the rare objective tumor response observed here and it is more likely that a combination that converted the cold tumors to hot was responsible.
Therefore, we finally consider whether the experimental AlloStim® priming alone or in combination with regorafenib contributed to the ICI objective response.
We hypothesized that if the restaging CT scan after AlloStim® immunotherapy reported as progressive disease (PD) by RECIST 1.1, was actually pseudoprogression due to ‘hot’ inflammation of the tumor lesions which would make them appear to be larger than the actual tumor burden, that ICI immunotherapy would elicit a rapid tumor debulking response due to resident infiltrating effector immune cell release from suppression.
The present subject was negative for serum IL-12 at baseline. After three cycles of experimental AlloStim® immunotherapy the subject seroconverted to IL-12 positivity, supporting that the host immune system was modulated. We previously reported that IL-12 positivity correlated with long-term survival after AlloStim® immunotherapy [63].
IL-12 is an effector cytokine that promotes anti-tumor immunity by activating an effector Th1 response, which is required for the activation of cytotoxic T and NK cells [64]. IL-12 promotes production of IFN-γ which acts to upregulate PD-L1 in the tumor microenvironment (TME), which may make these tumors more susceptible to anti-PD-L1 ICI immunotherapy [65,66,67].
The presence of IL-12 can have many beneficial anti-tumor effects, including: increasing production of IFN-γ from NK and T cells [68]; stimulation of growth and cytotoxicity of activated NK cells and CD8+ and CD4+ T cells [69], shifting the Th1/Th2 balance in favor of the Th1 phenotype [70]; induction of antiangiogenic cytokine and chemokine production [71]; remodeling of the peritumoral extracellular matrix and tumor stroma [72], reprogramming of myeloid-derived suppressor cells [73], and increasing expression of MHC class I molecules necessary of cytolytic T-lymphocyte (CTL) recognition of tumor cells [74].
Soluble heat shock protein (HSP)-70 was also detected in the serum after AlloStim® administration. HSP-70 is a stress-inducible chaperone that is overexpressed within tumor cells, including CRC [75]. The finding of HSP-70 in serum suggests that tumor cells have been killed in a manner where the cell membrane is disrupted (immunological cell death), releasing the HSP along with danger signals into the tumor microenvironment. Hsp70 extracellular function is believed to be immunogenic and extracellular Hsp70 can serve as an adjuvant to activate the innate immune system [76] and can eventually lead to tumor-specific adaptive immunity [77]. Endogenous HSP chaperone all tumor cell antigens, including self- and neo-antigens. Tumors accumulate mutations that can cause tumor-specific neo-antigen expression. Since these neo-antigens are intracellular, they may not have been previously exposed to the immune system, as the tumors sequester these neoantigens. Thus the presence of soluble HSP-70 supports that AlloStim® modified the TME in a manner that caused tumor lysis and release of chaperoned neoantigens. Exposure of tumor neoantigens to the immune system increases responsiveness to ICI [78].
The mechanism of action of AlloStim® is also consistent with the conclusion that an inflammatory cold to hot conversion occurred which caused the dramatic 73% increase in target lesion size by RECIST 1.1. The immune systems of patients with metastatic cancers are dysregulated resulting in a shift toward Th2 dominance [79,80,81]. AlloStim® experimental immunotherapy modulates the dysregulated immune systems of these patients to a Th1 dominance using a strategy of allo-priming [82]. The STIMVAX protocol incorporated a first series of intradermal injections of AlloStim®. The host rejection of the intentionally mis-matched AlloStim® cells shortly after administration results in increased titers of allo-specific Th1 and CTL cells, modulating the resident Th1/Th2 balance.
These allo-specific cells elicited after intradermal injections are non-specifically activated by cytokine release after intravenous infusion of AlloStim® through a bystander activation mechanism [83]. Activated T-cells can extravasate and local inflammation attracts these cells into tissues and sites of inflammation, including tumors [84]. Thus, the intravenous infusion of AlloStim® after allo-priming can convert “cold” tumors into “hot” tumors with extensive infiltration of Th1/CTL memory cells, which could possibly account for the 73% increase in target lesion size.
Inflamed tumor lesions can enlarge and appear as PD by RECIST 1.1. However, the putative anti-tumor mechanism leading to tumor debulking immunity may take several additional months before a radiological response can be detected and patients are often removed from the treatment protocols before a later assessment can be conducted.
We hypothesized that based on the mechanism of action of AlloStim® that radiological progression after 3 cycles likely represents a beneficial immune response that has primed the tumor lesions for an eventual debulking anti-tumor response. In order to support this hypothesis, we administered a short course of combination ICI immunotherapy. Since pMMR/MSS mCRC is known not to be responsive to ICI immunotherapy, we predicted that if a rapid tumor debulking response were observed, this would provide evidence supporting that the tumor lesions had been previously primed with infiltrating effector immune cells.
Conclusion
The available evidence makes it appear likely that AlloStim® played a role in eliciting the objective response observed after regorafenib and combination ICI immunotherapy.
The rare objective response in this case provides support for further investigation of the combination of AlloStim® combined with ICI immunotherapy with/or without regorafenib in ICI resistant patients.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- bid:
-
Twice a day
- BSC:
-
Basic supportive care
- CRC:
-
Colorectal cancer
- CS:
-
Corticosteroid
- CSF1R:
-
Colony stimulating factor receptor-1
- CT:
-
Computed tomography
- CTL:
-
Cytolytic T-lymphocyte
- CTLA4:
-
Cytotoxic T-lymphocyte associated protein 4
- CXCR3:
-
C-X-C motif chemokine receptor-3
- DC1:
-
Dendritic cell type 1
- dMMR:
-
Deficient DNA mismatch repair
- DNA:
-
Deoxyribonucleic acid
- ECOG:
-
Eastern Cooperative Oncology Group
- FDA:
-
Food and drug administration
- FGFR1,:
-
Epidermal growth factor receptor-1
- FGFR2,:
-
Epidermal growth factor receptor-2
- FOLFIRI:
-
Folinic acid, fluorouracil and irinotecan
- FOLFOX:
-
Folinic acid, fluorouracil and oxaliplatin
- GVHD:
-
Graft vs. host disease
- GVT:
-
Graft vs. tumor
- ICI:
-
Immune checkpoint inhibitor
- ID:
-
Intradermal
- IFN:
-
Interferon
- IL:
-
Interleukin
- irRC:
-
Immune related response criteria
- IV:
-
Intravenous
- LAG:
-
Lymphocyte activation gene
- LAR:
-
Low anterior resection
- mCRC:
-
Metastatic colorectal cancer
- MSI-H:
-
Microsatellite instability high
- MSS:
-
Microsatellite stable
- NK:
-
Natural killer cell
- OS:
-
Overall survival
- PBMC:
-
Peripheral blood mononuclear cell
- PD:
-
Progressive disease
- PD-1:
-
Programmed death receptor-1
- PDGFR:
-
Platelet derived growth factor receptor
- PD-L1:
-
Programmed death receptor-ligand 1
- PFS:
-
Progression free survival
- pMMR:
-
Proficient DNA mismatch repair
- POLD1:
-
Polymerase delta-1
- POLE:
-
Polymerase epsilon
- PR:
-
Partial response
- RAF1:
-
Raf-1 proto-oncogene, serine/threonine kinase
- RAS:
-
Rat sarcoma gene
- RECIST:
-
Response evaluation in solid tumors
- s/p:
-
Status post
- SD:
-
Stable Disease
- STAT3:
-
Signal transducer and activator of transcription 3
- TH1:
-
T-helper type 1
- TH2:
-
T-helper type 2
- tid:
-
Three times a day
- TIE2:
-
TEK tyrosine kinase, endothelial
- TIL:
-
Tumor infiltrating lymphocytes
- TMB:
-
Tumor mutational burden
- TME:
-
Tumor microenvironment
- USA:
-
United States of America
- VEGF:
-
Vascular endothelial growth factor
- VEGFR:
-
Vascular endothelial growth factor receptor
- XRT:
-
Radiation therapy
References
Andre T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med. 2020;383:2207–18.
Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill A, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36:773–9.
Oliveira AF, Bretes L, Furtado I. Review of PD-1/PD-L1 Inhibitors in Metastatic dMMR/MSI-H Colorectal Cancer. Front Oncol. 2019;9:396.
Oh CR, Kim JE, Hong YS, Kim SY, Ahn JB, Baek JY, Lee MA, Kang MJ, Cho SH, Beom SH, Kim TW. Phase II study of durvalumab monotherapy in patients with previously treated microsatellite instability-high/mismatch repair-deficient or POLE-mutated metastatic or unresectable colorectal cancer. Int J Cancer. 2022;150:2038–45.
Mulet-Margalef N, Linares J, Badia-Ramentol J, Jimeno M, Sanz Monte C, Manzano Mozo JL, et al. Challenges and Therapeutic Opportunities in the dMMR/MSI-H Colorectal Cancer Landscape. Cancers (Basel). 2023;15(4):1022. https://doi.org/10.3390/cancers15041022.
Lumish MA, Cercek A. Immunotherapy for the treatment of colorectal cancer. J Surg Oncol. 2021;123:760–74.
Gorzo A, Galos D, Volovat SR, Lungulescu CV, Burz C, Sur D. Landscape of Immunotherapy Options for Colorectal Cancer: Current Knowledge and Future Perspectives beyond Immune Checkpoint Blockade. Life (Basel). 2022;12(2):229.
Kalyan A, Kircher S, Shah H, Mulcahy M, Benson A. Updates on immunotherapy for colorectal cancer. J Gastrointest Oncol. 2018;9:160–9.
Kim GP, Colangelo LH, Wieand HS, Paik S, Kirsch IR, Wolmark N, Allegra CJ, National Cancer I. Prognostic and predictive roles of high-degree microsatellite instability in colon cancer: a National Cancer Institute-National Surgical Adjuvant Breast and Bowel Project Collaborative Study. J Clin Oncol. 2007;25:767–72.
Manz SM, Losa M, Fritsch R, Scharl M. Efficacy and side effects of immune checkpoint inhibitors in the treatment of colorectal cancer. Therap Adv Gastroenterol. 2021;14:17562848211002018.
Ganesh K, Stadler ZK, Cercek A, Mendelsohn RB, Shia J, Segal NH, Diaz LA Jr. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. 2019;16:361–75.
Matteucci L, Bittoni A, Gallo G, Ridolfi L, Passardi A. Immunocheckpoint Inhibitors in Microsatellite-Stable or Proficient Mismatch Repair Metastatic Colorectal Cancer: Are We Entering a New Era? Cancers (Basel). 2023;15(21):5189.
Pecci F, Cantini L, Bittoni A, Lenci E, Lupi A, Crocetti S, Giglio E, Giampieri R, Berardi R. Beyond microsatellite instability: evolving strategies integrating immunotherapy for microsatellite stable colorectal cancer. Curr Treat Options Oncol. 2021;22:69.
Bai J, Chen H, Bai X. Relationship between microsatellite status and immune microenvironment of colorectal cancer and its application to diagnosis and treatment. J Clin Lab Anal. 2021;35: e23810.
Wang L, Geng H, Liu Y, Liu L, Chen Y, Wu F, Liu Z, Ling S, Wang Y, Zhou L. Hot and cold tumors: Immunological features and the therapeutic strategies. MedComm. 2020;2023(4): e343.
Schrock AB, Ouyang C, Sandhu J, Sokol E, Jin D, Ross JS, Miller VA, Lim D, Amanam I, Chao J, et al. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer. Ann Oncol. 2019;30:1096–103.
Ooki A, Shinozaki E, Yamaguchi K. Immunotherapy in colorectal cancer: current and future strategies. J Anus Rectum Colon. 2021;5:11–24.
Thomas J, Leal A, Overman MJ. Clinical Development of immunotherapy for deficient mismatch repair colorectal cancer. Clin Colorectal Cancer. 2020;19:73–81.
Klempner SJ, Fabrizio D, Bane S, Reinhart M, Peoples T, Ali SM, Sokol ES, Frampton G, Schrock AB, Anhorn R, Reddy P. Tumor mutational burden as a predictive biomarker for response to immune checkpoint inhibitors: a review of current evidence. Oncologist. 2020;25:e147–59.
Manca P, Corti F, Intini R, Mazzoli G, Miceli R, Germani MM, Bergamo F, Ambrosini M, Cristarella E, Cerantola R, et al. Tumour mutational burden as a biomarker in patients with mismatch repair deficient/microsatellite instability-high metastatic colorectal cancer treated with immune checkpoint inhibitors. Eur J Cancer. 2023;187:15–24.
Li J, Xu X. Immune checkpoint inhibitor-based combination therapy for colorectal cancer: an overview. Int J Gen Med. 2023;16:1527–40.
Lizardo DY, Kuang C, Hao S, Yu J, Huang Y, Zhang L. Immunotherapy efficacy on mismatch repair-deficient colorectal cancer: From bench to bedside. Biochim Biophys Acta Rev Cancer. 2020;1874: 188447.
Liu N, Shan F, Ma M. Strategic enhancement of immune checkpoint inhibition in refractory Colorectal Cancer: Trends and future prospective. Int Immunopharmacol. 2021;99: 108017.
Har-Noy M, Slavin S. The anti-tumor effect of allogeneic bone marrow/stem cell transplant without graft vs. host disease toxicity and without a matched donor requirement? Med Hypotheses. 2008;70:1186–92.
Har-Noy M, Zeira M, Weiss L, Fingerut E, Or R, Slavin S. Allogeneic CD3/CD28 cross-linked Th1 memory cells provide potent adjuvant effects for active immunotherapy of leukemia/lymphoma. Leuk Res. 2009;33:525–38.
LaCasse CJ, Janikashvili N, Larmonier CB, Alizadeh D, Hanke N, Kartchner J, Situ E, Centuori S, Har-Noy M, Bonnotte B, et al. Th-1 lymphocytes induce dendritic cell tumor killing activity by an IFN-gamma-dependent mechanism. J Immunol. 2011;187:6310–7.
Janikashvili N, LaCasse CJ, Larmonier C, Trad M, Herrell A, Bustamante S, Bonnotte B, Har-Noy M, Larmonier N, Katsanis E. Allogeneic effector/memory Th-1 cells impair FoxP3+ regulatory T lymphocytes and synergize with chaperone-rich cell lysate vaccine to treat leukemia. Blood. 2011;117:1555–64.
Ma K, Jin Q, Wang M, Li X, Zhang Y. Research progress and clinical application of predictive biomarker for immune checkpoint inhibitors. Expert Rev Mol Diagn. 2019;19:517–29.
Zhang Y, Zheng L. Tumor immunotherapy based on tumor-derived heat shock proteins (Review). Oncol Lett. 2013;6:1543–9.
Srivastava PK, Callahan MK, Mauri MM. Treating human cancers with heat shock protein-peptide complexes: the road ahead. Expert Opin Biol Ther. 2009;9:179–86.
Hammerich L, Bhardwaj N, Kohrt HE, Brody JD. In situ vaccination for the treatment of cancer. Immunotherapy. 2016;8:315–30.
Felzmann T, Huttner KG, Breuer SK, Wimmer D, Ressmann G, Wagner D, Paul P, Lehner M, Heitger A, Holter W. Semi-mature IL-12 secreting dendritic cells present exogenous antigen to trigger cytolytic immune responses. Cancer Immunol Immunother. 2005;54:769–80.
Waxman ES, Lee Gerber D. Pseudoprogression and Immunotherapy Phenomena. J Adv Pract Oncol. 2020;11:723–31.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, Maio M, Binder M, Bohnsack O, Nichol G, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20.
Hales RK, Banchereau J, Ribas A, Tarhini AA, Weber JS, Fox BA, Drake CG. Assessing oncologic benefit in clinical trials of immunotherapy agents. Ann Oncol. 2010;21:1944–51.
Ippolito D, Maino C, Ragusi M, Porta M, Gandola D, Franzesi CT, Giandola TP, Sironi S. Immune response evaluation criteria in solid tumors for assessment of atypical responses after immunotherapy. World J Clin Oncol. 2021;12:323–34.
Borcoman E, Nandikolla A, Long G, Goel S, Le Tourneau C. Patterns of Response and Progression to Immunotherapy. Am Soc Clin Oncol Educ Book. 2018;38:169–78.
Morse MA, Overman MJ, Hartman L, Khoukaz T, Brutcher E, Lenz HJ, Atasoy A, Shangguan T, Zhao H, El-Rayes B. Safety of Nivolumab plus Low-Dose ipilimumab in previously treated microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer. Oncologist. 2019;24:1453–61.
Almquist DR, Ahn DH, Bekaii-Saab TS. The Role of immune checkpoint inhibitors in colorectal adenocarcinoma. BioDrugs. 2020;34:349–62.
Wang L, Huang C. Application of immune checkpoint inhibitors in colorectal cancer. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2021;46:894–9.
Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182–91.
Chung KY, Gore I, Fong L, Venook A, Beck SB, Dorazio P, Criscitiello PJ, Healey DI, Huang B, Gomez-Navarro J, Saltz LB. Phase II study of the anti-cytotoxic T-lymphocyte-associated antigen 4 monoclonal antibody, tremelimumab, in patients with refractory metastatic colorectal cancer. J Clin Oncol. 2010;28:3485–90.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54.
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65.
Salem ME, Puccini A, Grothey A, Raghavan D, Goldberg RM, Xiu J, Korn WM, Weinberg BA, Hwang JJ, Shields AF, et al. Landscape of tumor mutation load, mismatch repair deficiency, and PD-L1 expression in a large patient cohort of gastrointestinal cancers. Mol Cancer Res. 2018;16:805–12.
Chen EX, Jonker DJ, Loree JM, Kennecke HF, Berry SR, Couture F, Ahmad CE, Goffin JR, Kavan P, Harb M, et al. Effect of Combined Immune Checkpoint Inhibition vs Best Supportive Care Alone in Patients With Advanced Colorectal Cancer: The Canadian Cancer Trials Group CO.26 Study. JAMA Oncol. 2020;6:831–8.
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et al. PD-1 Blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–20.
Parseghian CM, Patnana M, Bhosale P, Hess KR, Shih YT, Kim B, Kopetz S, Overman MJ, Varadhachary GR, Javle M, et al. Evaluating for pseudoprogression in colorectal and pancreatic tumors treated with immunotherapy. J Immunother. 2018;41:284–91.
El Hajj J, Reddy S, Verma N, Huang EH, Kazmi SM. Immune Checkpoint Inhibitors in pMMR/MSS Colorectal Cancer. J Gastrointest Cancer. 2023;54:1017–30.
Lin KX, Istl AC, Quan D, Skaro A, Tang E, Zheng X. PD-1 and PD-L1 inhibitors in cold colorectal cancer: challenges and strategies. Cancer Immunol Immunother. 2023;72:3875–93.
Huyghe N, Baldin P, Van den Eynde M. Immunotherapy with immune checkpoint inhibitors in colorectal cancer: what is the future beyond deficient mismatch-repair tumours? Gastroenterol Rep (Oxf). 2020;8:11–24.
Tintelnot J, Stein A. Immunotherapy in colorectal cancer: Available clinical evidence, challenges and novel approaches. World J Gastroenterol. 2019;25:3920–8.
Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouche O, Mineur L, Barone C, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–12.
Lucidarme O, Wagner M, Gillard P, Kim S, Bachet JB, Rousseau B, Mazard T, Louvet C, Chibaudel B, Cohen R, et al. RECIST and CHOI criteria in the evaluation of tumor response in patients with metastatic colorectal cancer treated with regorafenib, a prospective multicenter study. Cancer Imaging. 2019;19:85.
Akin Telli T, Bregni G, Vanhooren M, Saude Conde R, Hendlisz A, Sclafani F. Regorafenib in combination with immune checkpoint inhibitors for mismatch repair proficient (pMMR)/microsatellite stable (MSS) colorectal cancer. Cancer Treat Rev. 2022;110: 102460.
Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, Yoshii T, Kotani D, Tamura H, Mikamoto Y, et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase Ib Trial (REGONIVO, EPOC1603). J Clin Oncol. 2020;38:2053–61.
Wang C, Chevalier D, Saluja J, Sandhu J, Lau C, Fakih M. Regorafenib and Nivolumab or Pembrolizumab Combination and Circulating Tumor DNA Response Assessment in Refractory Microsatellite Stable Colorectal Cancer. Oncologist. 2020;25:e1188–94.
Wang F, He MM, Yao YC, Zhao X, Wang ZQ, Jin Y, Luo HY, Li JB, Wang FH, Qiu MZ, et al. Regorafenib plus toripalimab in patients with metastatic colorectal cancer: a phase Ib/II clinical trial and gut microbiome analysis. Cell Rep Med. 2021;2: 100383.
Yang K, Han L, Wu S, Qu X, Li Q, Zhao C, Zhou J, Jin X, Wang Y, Yan D, et al. Real-world outcomes of regorafenib combined with immune checkpoint inhibitors in patients with advanced or metastatic microsatellite stable colorectal cancer: A multicenter study. Cancer Immunol Immunother. 2022;71:1443–51.
Fakih M, Raghav KPS, Chang DZ, Larson T, Cohn AL, Huyck TK, Cosgrove D, Fiorillo JA, Tam R, D’Adamo D, et al. Regorafenib plus nivolumab in patients with mismatch repair-proficient/microsatellite stable metastatic colorectal cancer: a single-arm, open-label, multicentre phase 2 study. EClinicalMedicine. 2023;58: 101917.
Maslov DV, Tawagi K, Kc M, Simenson V, Yuan H, Parent C, Bamnolker A, Goel R, Blake Z, Matrana MR, Johnson DH. Timing of steroid initiation and response rates to immune checkpoint inhibitors in metastatic cancer. J Immunother Cancer. 2021;9(7):e002261.
Har-Noy M, Lausoontornsiri W, Or R, Katsanis E. Response of Her2+ breast cancer patients to allogeneic cell immunotherapy. J Clin Oncol. 2012;30(suppl): e13013.
Lu X. Impact of IL-12 in Cancer. Curr Cancer Drug Targets. 2017;17:682–97.
Yuan W, Deng D, Jiang H, Tu C, Shang X, He H, Niu R, Dong J. Hyperresponsiveness to interferon gamma exposure as a response mechanism to anti-PD-1 therapy in microsatellite instability colorectal cancer. Cancer Immunol Immunother. 2019;68:257–68.
Kikuchi T, Mimura K, Okayama H, Nakayama Y, Saito K, Yamada L, Endo E, Sakamoto W, Fujita S, Endo H, et al. A subset of patients with MSS/MSI-low-colorectal cancer showed increased CD8(+) TILs together with up-regulated IFN-gamma. Oncol Lett. 2019;18:5977–85.
Yuan W, Deng D, Li H, Hu X, Shang X, Hou X, Jiang H, He H. IFNgamma/PD-L1 Signaling Improves the Responsiveness of Anti-PD-1 Therapy in Colorectal Cancer: An in vitro Study. Onco Targets Ther. 2021;14:3051–62.
Otani T, Nakamura S, Toki M, Motoda R, Kurimoto M, Orita K. Identification of IFN-gamma-producing cells in IL-12/IL-18-treated mice. Cell Immunol. 1999;198:111–9.
Zeh HJ 3rd, Hurd S, Storkus WJ, Lotze MT. Interleukin-12 promotes the proliferation and cytolytic maturation of immune effectors: implications for the immunotherapy of cancer. J Immunother Emphasis Tumor Immunol. 1993;14:155–61.
Trinchieri G, Wysocka M, D’Andrea A, Rengaraju M, Aste-Amezaga M, Kubin M, Valiante NM, Chehimi J. Natural killer cell stimulatory factor (NKSF) or interleukin-12 is a key regulator of immune response and inflammation. Prog Growth Factor Res. 1992;4:355–68.
Angiolillo AL, Sgadari C, Tosato G. A role for the interferon-inducible protein 10 in inhibition of angiogenesis by interleukin-12. Ann N Y Acad Sci. 1996;795:158–67.
Kerkar SP, Leonardi AJ, van Panhuys N, Zhang L, Yu Z, Crompton JG, Pan JH, Palmer DC, Morgan RA, Rosenberg SA, Restifo NP. Collapse of the tumor stroma is triggered by IL-12 induction of Fas. Mol Ther. 2013;21:1369–77.
Kerkar SP, Goldszmid RS, Muranski P, Chinnasamy D, Yu Z, Reger RN, Leonardi AJ, Morgan RA, Wang E, Marincola FM, et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J Clin Invest. 2011;121:4746–57.
Suzuki S, Umezu Y, Saijo Y, Satoh G, Abe Y, Satoh K, Nukiwa T. Exogenous recombinant human IL-12 augments MHC class I antigen expression on human cancer cells in vitro. Tohoku J Exp Med. 1998;185:223–6.
Kanazawa Y, Isomoto H, Oka M, Yano Y, Soda H, Shikuwa S, Takeshima F, Omagari K, Mizuta Y, Murase K, et al. Expression of heat shock protein (Hsp) 70 and Hsp 40 in colorectal cancer. Med Oncol. 2003;20:157–64.
Sherman MY, Gabai VL. Hsp70 in cancer: back to the future. Oncogene. 2015;34:4153–61.
Saxena M, van der Burg SH, Melief CJM, Bhardwaj N. Therapeutic cancer vaccines. Nat Rev Cancer. 2021;21(6):360–78.
Ma S, Chee J, Fear VS, Forbes CA, Boon L, Dick IM, Robinson BWS, Creaney J. Pre-treatment tumor neo-antigen responses in draining lymph nodes are infrequent but predict checkpoint blockade therapy outcome. Oncoimmunology. 2020;9:1684714.
Pellegrini P, Berghella AM, Del Beato T, Cicia S, Adorno D, Casciani CU. Disregulation in TH1 and TH2 subsets of CD4+ T cells in peripheral blood of colorectal cancer patients and involvement in cancer establishment and progression. Cancer Immunol Immunother. 1996;42:1–8.
Sato M, Goto S, Kaneko R, Ito M, Sato S, Takeuchi S. Impaired production of Th1 cytokines and increased frequency of Th2 subsets in PBMC from advanced cancer patients. Anticancer Res. 1998;18:3951–5.
Shurin MR, Lu L, Kalinski P, Stewart-Akers AM, Lotze MT. Th1/Th2 balance in cancer, transplantation and pregnancy. Springer Semin Immunopathol. 1999;21:339–59.
Har-Noy M, Or R. Allo-priming as a universal anti-viral vaccine: protecting elderly from current COVID-19 and any future unknown viral outbreak. J Transl Med. 2020;18:196.
Paprckova D, Salyova E, Michalik J, Stepanek O. Bystander activation in memory and antigen-inexperienced memory-like CD8 T cells. Curr Opin Immunol. 2023;82: 102299.
Newton P, O’Boyle G, Jenkins Y, Ali S, Kirby JA. T cell extravasation: demonstration of synergy between activation of CXCR3 and the T cell receptor. Mol Immunol. 2009;47:485–92.
Acknowledgements
The authors wish to thank Axella Clinical Research (CRO) for assistance in coordinating data collection and research blood samples, Dr. Xiaochuan Yang and Eirini Topouzi for analyzing blood samples and assisting with preparation of figures. Dr. Elena Fingerut for managing the manufacturing of the study drug in accordance with good manufacturing practices and Thu Bui for managing blood donor collection pursuant to 21 CFR 1271 and the distribution of the frozen investigational product to the clinical site.
Funding
Mirror Biologics, Inc. is the sponsor of ITL-032-MCRC3-STIMVAX Phase IIB clinical trial and has funded the research.
Author information
Authors and Affiliations
Contributions
MHN wrote the manuscript with input and review from other authors. AH conceived the design of the study and was responsible for implementing the protocol and for patient care. DW reviewed the longitudinal CT scans and determined the RECIST 1.1 conclusions and selected the CT scan images.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Salus IRB, 2111 W. Braker Lane, Ste 100, Austin, TX 78758, serves as the central ethics committee for this study (ITL-032-MCRC3-STIMVAX). Informed consent was obtained from all individual participants included in the study.
Consent for publication
The patient informed consent form provided consent to publish non-identifiable results.
Competing interests
MHN is the inventor of the investigational AlloStim® product and is the founder of Mirror Biologics, Inc. which sponsored the clinical study. AH and DG declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hirschfeld, A., Gurell, D. & Har-Noy, M. Objective response after immune checkpoint inhibitors in a chemotherapy-refractory pMMR/MSS metastatic rectal cancer patient primed with experimental AlloStim® immunotherapy. transl med commun 9, 15 (2024). https://doi.org/10.1186/s41231-024-00174-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41231-024-00174-y