Abstract
Background
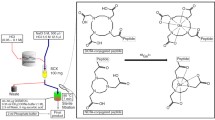
The widespread acceptance and application of 68Ga-PET depends on our ability to develop radiopharmaceuticals that can be prepared in a convenient and suitable manner. A kit-type labelling protocol provides such characteristics and requires chelators that can be radiolabelled under exceptionally mild conditions. Recently the DATA chelators have been introduced that fulfil these requirements. In continuing their development, the synthesis and radiolabelling of the first DATA bifunctional chelator (BFC) and peptide conjugate are described.
Results
A BFC derived from the DATA ligand (2,2’-(6-((carboxymethyl)amino)-1,4-diazepane-1,4-diyl)diacetic acid) has been synthesised in five steps from simple building blocks, with an overall yield of 8 %. DATAM5-3tBu (5-[1,4-Bis-tert-butoxycarbonylmethyl-6-(tert-butoxycarbonylmethyl-methyl-amino)-[1, 4]diazepan-6-yl]-pentanoic acid) has been coupled to [DPhe1][Tyr3]-octreotide (TOC) and the resulting peptide conjugate (DATATOC) radiolabelled with purified 68Ga derived via four different 68Ge/68Ga generator post-processing (PP) methods. The stability and lipophilicity of the radiotracer have been assessed and a kit-type formulation for radiolabelling evaluated. 68Ga-DATATOC has been prepared with a > 95 % radiochemical yield (RCY) within 1 (fractionated and acetone-PP) and 10 min (ethanol- and NaCl-PP) at 23 °C (pH 4.2–4.9, 13 nmol). The radiolabelled peptide is stable in the presence of human serum. Lipophilicity of 68Ga-DATATOC was calculated as logP = −3.2 ± 0.3, with a HPLC retention time (t R = 10.4 min) similar to 68Ga-DOTATOC (logP = −2.9 ± 0.4, t R = 10.3 min). Kit-type labelling from a lyophilised solid using acetone-PP based labelling achieves > 95 % RCY in 10 min at 23 °C.
Conclusions
The favourable labelling properties of the DATA chelators have been retained for DATATOC. High radiochemical purity can be achieved at 23 °C in less than 1 min and from a kit formulation. The speed, reliability, ease, flexibility and simplicity with which 68Ga-DATATOC can be prepared makes it a very attractive alternative to current standards.
Similar content being viewed by others
Background
The positron emitter 68Ga has a number of characteristics which make it a very attractive and promising radionuclide for PET imaging of disease and infection (Smith et al. 2013; Fani et al. 2008). In spite of this and numerous publications which provide support for its superiority, more established radionuclides and imaging modalities stand in its path (Buchmann et al. 2007; Tran et al. 2015). Interest in 68Ga-PET in Europe has grown over the last years, highlighted by the recent promotion of 68Ga -DOTATATE and -DOTATOC to orphan drug status in the United States (FDA Grants Orphan Drug Designation for 68Ga-DOTATOC. J Nucl Med 2014). 99mTc became the work-horse of nuclear medicine in part due to the development of SPECT radiopharmaceuticals which can be prepared in a simple kit-type manner (Roesch 2013). The development of chelators that permit a similar protocol for 68Ga complement the inherent advantages of the 68Ge/68Ga-generator, paving the way for realisation of its full potential and delivery of advanced imaging diagnostics world-wide (Mukherjee et al. 2014; Velikyan et al. 2008).
The last decade of 68Ga-radiopharmaceutical chemistry has been dominated by studies with DOTATOC and its derivatives for the diagnosis of neuroendocrine tumours (NETs) (Frilling et al. 2010). The omnipresence of DOTA derivatives in 68Ga-PET arose from its success in other imaging applications (MRI and optical imaging with lanthanides) and its ability to provide an acceptable labelling profile and sufficient complex stability with 68Ga (Notni et al. 2011; Boros et al. 2010). Optimisation of radiolabelling protocols and the development of labelling modules have simplified radiopharmaceutical preparation, but the desire for further development remains (Ocak et al. 2010). The major inherent disadvantage of DOTA derivatives is the relatively harsh conditions required for radiolabelling (80–95 °C, 5–10 min, pH 3–4) that impose a number of limitations (Notni et al. 2011; Boros et al. 2010). Chelators which can be labelled quickly at room temperature would simplify labelling further and offer the potential for kit-type labelling akin to the prestigious 99mTc kits. Temperature/pH sensitive targeting vectors (TVs) would benefit from these new chelators in particular, and widen the portfolio of 68Ga-based diagnostics. In recent times, there has been greater focus on the development of more efficient hexadentate chelators, and bifunctional derivatives of NOTA, TRAP, NOPO, DEDPA, CP256, HBED have been described that chelate 68Ga(III) rapidly at room temperature (Notni et al. 2011; Fani et al. 2012; Simeček et al. 2012; Boros et al. 2012; Berry et al. 2011; Eder et al. 2008).
The potential for kit-type labelling of biomolecule conjugates has been alluded to on numerous occasions, but a protocol which can achieve acceptable yields from a lyophilised solid at ambient temperature remains ‘the final frontier’ (Mukherjee et al. 2014; Velikyan et al. 2008 Asti et al. 2015; Waengler et al. 2011). The DATA chelators rapidly form stable complexes with 68Ga under exceptionally mild conditions befitting kit-type labelling (Waldron et al. 2013; Parker & Waldron 2013; Parker et al. 2013). The ligand DATAM (Fig. 1) for instance, can enable radiochemical yields (RCYs) greater than 97 % at 23 °C in under 1 min (Seemann et al. 2015a). Earlier work showed that it is possible to label DATA chelators over the pH range 4–7, however for the purposes of this work it was decided that only optimum labelling conditions would be used in line with the desire to develop a room temperature kit-type labelling protocol (Waldron et al. 2013; Seemann et al. 2015a).
The main objective of this work has been to demonstrate the potential of a DATA conjugate towards development of kit-type labelling in a setting that holds considerable practical interest. The peptide TOC has been extensively studied with other chelators (DOTA, NODAGA, DTPA, DFO) in both imaging and therapeutic modalities, and has significant clinical and commercial importance (Lin et al. 2013; Eisenwiener et al. 2002; Dumont et al. 2011; Fani et al. 2011; Ugur et al. 2002). Hence, DATA conjugates were envisaged to allow comparative analysis.
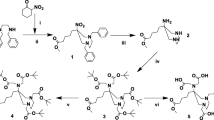
The first step was to develop and synthesise a novel DATA bifunctional prochelator (DATAM5-3tBu) that permits convenient amide conjugation to the N-terminus of TOC. A bifunctional derivative of DATAM has been synthesised and conjugated to protected TOC using standard methods (Fig. 1). An initial radiolabelling evaluation of the conjugate (DATATOC, Fig. 1) has been carried out at ambient temperature, and a kit-type formulation tested.
Methods
Reagents were purchased from Sigma-Aldrich® or Merck® and used without further purification. Purite® water used was filtered through a Millex® Millipore filter membrane (0.54 μm). Reaction progress was monitored using silica TLC-plates (silica 60 F254 4.5 × 4.5 cm, Merck) and visualised with UV254nm and/or KMnO4. Column chromatography was performed with silica gel 60 (Fisher Scientific®; 0.04–0.063 nm). NMR spectra (1H, 13C, HSQC, HMBC) were recorded on an Avance III HD 400 (Bruker, United States). Chemical shifts are given in ppm. MS (ESI) were performed with a Thermo Quest Navigator Instrument (Thermo Electron). Mass spec results are given as m/z in g/mol. HPLC was performed with a metal-free Dionex ICS-5000 system with a quaternary pump, an AS-50 auto sampler, UV/vis detector and automated fraction collector AFC-3000.
Synthesis
The chemical identity of synthesised compounds has been confirmed by 1H-, 13C-NMR and HR MS with the exception of TOC conjugates, which have been characterised by HPLC and HRMS. 1H-, 13C-NMR and HRMS data for compounds prior to TOC conjugation are provided in the S.I. (Fig. 2).
5-(1,4-Dibenzyl-6-nitro-[1, 4]diazepan-6-yl)-pentanoic acid methyl ester (1) 2-Nitrocyclohexanone (0.608 g, 4.3 mmol) was added to Amberlyst A21 (1.216 g, 2 mass equivalents) in EtOH and stirred for 2 h at 60 °C under argon. N,N′-Dibenzyl-ethylenediamine (1.020 g, 4.3 mmol) and paraformaldehyde (0.446 g, 14.9 mmol) were added and the reaction stirred at 60 °C overnight. The mixture was filtered through Celite®, and solvent removed under reduced pressure. The resulting residue was re-dissolved in CHCl3 (40 mL) and washed successively with aqueous K2CO3 solution (2 × 30 mL, 0.1 M) and H2O (30 mL), dried over MgSO4, filtered and solvent removed under reduced pressure. Purification by silica gel column chromatography (DCM) afforded the title compound as a yellow oil (1.607 g, 85 %). Rf = 0.80 (DCM).
5-(1,4-Dibenzyl-6-nitro-[1,4]diazepan-6-yl)-pentanoic acid methyl ester (2) A catalytic amount of Pd(OH)2/C and acetic acid (50 μL, 0.87 mmol) was added to the protected triamine 1 (0.10 g, 0.29 mmol) in MeOH (20 mL), and the mixture agitated under an atmosphere of hydrogen for 3 h (1 atm H2). TLC (DCM) was used to confirm complete reduction of the nitro group and cleavage of the benzyl N-substituents. Pd(OH)2/C was removed using a Celite® filter. The solvent was removed under reduced pressure to afford a yellow oil. (0.065 g, 97 %)
5-[1,4-Bis-tert-butoxycarbonylmethyl-6-(tert-butoxycarbonylmethyl-amino)-[1,4]diazepan-6-yl]-pentanoic acid methyl ester (3) tert-Butyl-bromoacetate (0.567 g, 2.91 mmol) was added to 2 (0.208 g, 0.91 mmol) and K2CO3 (0.377 g, 2.73 mmol) in MeCN (25 mL), and the mixture stirred for 24 h at 368 K under an atmosphere of argon. The reaction was monitored by TLC (hexane/ethyl acetate; 1:1) for formation of the tetra-alkylated derivative. The solvent was removed under reduced pressure, and the resulting oil re-dissolved in CHCl3 (25 mL) and washed successively with aqueous K2CO3 solution (2 × 25 mL, 0.1 M) and H2O (25 mL), dried over MgSO4, filtered and solvent removed under reduced pressure. Purification by silica gel column chromatography (hexane/ethyl acetate, 2:1 → 1:1) afforded a yellow oil (0.229 g, 44 %). Rf = 0.35 (hexane/ethyl acetate; 2:1).
5-[1,4-Bis-tert-butoxycarbonylmethyl-6-(tert-butoxycarbonylmethyl-methyl-amino)-[1,4]diazepan-6-yl]-pentanoic acid methyl ester (4) Iodomethane (0.023 g, 0.16 mmol) was added to 3 (0.104 g, 0.18 mmol) and K2CO3 (0.025 g, 0.18 mmol) in DCM/MeCN (3:1) cooled in an ice-bath. The reaction mixture was allowed to warm to room temperature and left overnight. The solvent was removed under reduced pressure and the resulting oil re-dissolved in CHCl3 (20 mL), filtered, and washed successively with aqueous K2CO3 solution (2 × 20 mL, 0.1 M) and H2O (20 mL), dried over MgSO4, filtered and solvent removed under reduced pressure. Purification by silica gel column chromatography (hexane/ethyl acetate, 3:1 → 2:1) afforded a yellow oil (0.043 g, 46 %). Rf = 0.38 (hexane/ethyl acetate; 2:1).
5-[1,4-Bis-tert-butoxycarbonylmethyl-6-(tert-butoxycarbonylmethyl-methyl-amino)-[1,4]diazepan-6-yl]-pentanoic acid (5) LiOH (0.009 g, 0.039 mmol) dissolved in H2O (0.5 mL) was added to 4 (0.010 g, 0.023 mmol) in THF (0.5 mL), and the mixture stirred at 298 K. The reaction was monitored using LC-ESI MS for ester cleavage. Once complete, the solvent was removed by lyophilisation. H2O (5 mL) was added and removed by lyophilisation, and the procedure repeated two more times. The resulting solid was washed with ice-cold DCM (0.5 mL), and dried in vacuo to afford a waxy yellow solid (0.009 g, 70 %).
Synthesis of DATATOC
Commercially available protected TOC [(D)Phe1,Tyr(tBu)3,D-Trp(Boc)4,Lys(Boc)5, Thr(tBu)6,8]-octreotide was coupled to 5 using N,N-diisopropylethylamine (DIPEA) and O-(benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate (HBTU). The protected TOC (0.024 g, 0.017 mmol), HBTU (0.0010 g, 0.029 mmol), DIPEA (0.0016 g, 0.12 mmol) and 5 (0.015 g, 0.026 mmol) were added to dry DMSO (2 mL) and stirred at ambient temperature for 4 h. The solvent was removed under reduced pressure, and the resulting residue dissolved in 1 mL MeCN. The precipitate was removed by centrifugation, and solvent removed by lyophilisation. The resulting oil was purified by HPLC (C18-RP; A = 0.1 % TFA in H2O; B = MeCN; 0–2 min 35 % A; linear gradient to 10 % A at 14 min, 14–24 min 10 % A). The fully protected product (6) eluted with a retention time of 21 min (0.014 g, 41 %). MS ES+ (m/z) found: 1957.1133 [M + H]+; C100H158N13O22S2 calcd for: 1957.1086.
Deprotecting of the chelator and TOC was performed by dissolving 6 (0.050 g, 2.6 × 10−5 mmol) in a mixture of TFA/water/triisopropylsilane (95:2.5:2.5) and stirring at ambient temperature overnight. Once complete, the solvent was removed by lyophilisation. H2O/MeCN (1 mL, 1:1) was added and removed by lyophilisation, and the procedure repeated two more times. Purification was performed by preparative HPLC (C18-RP; A = 0.1 % TFA in water and B = MeCN; linear gradient: 0 min 80 % A, 15 min 65 % A, tR (product) = 12 min). DATATOC was eluted with a retention time of 12 min (0.006 g, 76 %). HR MS ES+ (m/z) found: 1421.6259 [M + H]+; calcd. for C66H94N13O18S2: 1420.6281 (Fig. 3).
Radiochemistry
All radiochemical evaluations were conducted using chemicals of the highest available purity grade. Volumes were measured using an Eppendorf pipette. A TiO2-based 68Ge/68Ga generator (Cyclotron Co., Obninsk, Russia) was used for all radiochemical evaluations. Four post-processing (PP) methods were used to purify and pre-concentrate the radioactive eluate: fractionation, acetone-, ethanol- and NaCl-based. Procedures were carried out, and solutions required prepared, as detailed in the relevant publication (Zhernosekov et al. 2007; Mueller et al. 2012; Eppard et al. 2014; Breeman et al. 2005). pH was measured using a Mettler-Toledo, SevenEasy pH. Radio-TLC was performed using silica-60 TLC plates (Merck F254, 4.5 × 4.5 cm), and eluted using 0.1 M citrate buffer (pH 4). Eluted radio-TLC plates were analysed using a flat-bed imaging scanner (Instant Imager, Canberra Packard).
Radiolabelling
Experiments were carried out in triplicate, and the amount of DATATOC used was constant in each case (13 nmol) taken from a 1 mg/mL stock solution. The volume of eluate differed according to the post-processing used, but was diluted as necessary so that ~ 100 MBq 68Ga was used for radiolabelling in each instance. Radiolabelling experiments were maintained at 23 °C by means of a heater-shaker device (DITABIS MHR 11), which was also used to agitate (400 rpm) radiolabelling solutions. TLC samples (1 μL) were taken at 1, 3, 5 and 10 min. The optimised labelling conditions vary according to the PP method applied. The important differences and approach used in each case are summarised in Table 1.
Stability of 68Ga-DATATOC
Stability was assessed following formation of 68Ga-DATATOC using ethanol-PP 68Ga and performed in triplicate. The stability was assessed by incubating 68Ga-DATATOC (50 μL) in an excess of human serum (300 μL) at 37 °C and pH 7 (1.0 M phosphate buffered saline) for 2 h. Samples (1 μL) were taken at 30, 60, 90 and 120 min and analysed by radio-TLC.
Lipophilicity of 68Ga-DATATOC
The lipophilicity was determined using the shake-flask method (logP) and qualitatively by radio-HPLC relative to 68Ga-DOTATOC (Notni et al. 2013; Du et al. 1998). Radiolabels were prepared following acetone-PP. Analytical radio-HPLC (LiChrospher 100-RP-18EC, 1 mL/min) for lipophilicity studies was based on a gradient using H2O (A) and MeCN (B), each containing 0.1 % TFA. Mobile phase gradient: 0–1.5 min 1 % B, 1.5–10.5 min 99 % B, 11.5 min 99 % B, 12–15 min 1 % B. The HPLC was coupled to a UV (Hitachi L-7400) and a radioactivity (Gamma Raytest) detection system. HPLC grade solvents were used and degassed by sonication for 15–20 min prior to use.
Kit-type labelling
Formulations for labelling following fractionation and acetone-PP were used to investigate the possibility of kit-type labelling.
Acetone-PP: The precursor solution was prepared as per Table 1 and lyophilised into a vial suitable for labelling. The post-processed 68Ga was diluted to the labelling volume (1.4 mL) using H2O and added to the lyophilised solid. The mixture was agitated on a test-tube vortex for 2 s and left to stand at ambient temperature.
Fractionated 68 Ga: owing to the nature of the labelling media used, it was not possible to prepare a lyophilised solid. After addition of 68Ga, the labelling solution was agitated on a test-tube vortex for 2 s and left to stand at ambient temperature. After 10 min a 1 μL sample was extracted and analysed by radio-TLC.
Results
Cold-Synthesis
The synthesis of DATAM5-3tBu was successfully carried out in five-steps in an 8 % yield. A further two steps were required to afford DATATOC in a yield of 31 %.
Radiochemical evaluations
The optimised labelling of DATATOC using 68Ga PP by four common procedures is shown in Fig. 4. The labelling volume varies depending on the PP method used, and as a result the precursor concentration is not the same in each case. Radiochemical yields (RCYs given with ± SD, n = 3) greater than 95 % were achieved with each type of PP eluate within 10 min, and from a kit formulation. Radio-HPLC confirmed that the radiolabelled product was the same in each instance.
In human serum 98.7 % of the initially present 68Ga-DATATOC remained intact after 2 h. The lipophilicity of 68Ga-DATATOC and 68Ga-DOTATOC, determined using the shake-flask method, are logP = −3.22 ± 0.32 and −2.93 ± 0.37 respectively. The respective retention times from radio-HPLC are 10.4 and 10.3 min.
Discussion
The synthesis of DATAM5-3tBu followed the same synthetic pathway as that of DATAM, with the only modification being the type of reagent used in the first step. A similar synthetic route was reported for an AAZTA conjugate with the same linker functionality, and the product labelled with 68Ga (Manzoni et al. 2012). In our experience, the heptadentate AAZTA ligand itself is unsuitable for chelation of 68Ga due to the formation of multiple radiolabelled species (Baranyai et al. 2009). The spacer and linker moieties for conjugation are incorporated in the first step during formation of the 7-membered diazepine ring. A five-carbon spacer, attached to the quaternary carbon of the ring, possesses an acid which is orthogonally protected as an ester relative to the chelating acids, to ensure selective conjugation of TOC. The key step is the N-methylation (step 4), necessary to prevent an internal cyclisation with an adjacent acetate, where care is required to prevent formation of a quaternary amine. A suitably protected TOC derivative was conjugated in solution, with subsequent deprotecting of the chelator and peptide occurring simultaneously.
There are four commonly used 68Ga generators and four main PP methods (acetone-, ethanol-, NaCl- based and fractionation) (Zhernosekov et al. 2007; Mueller et al. 2012; Eppard et al. 2014; Breeman et al. 2005). Each generator and PP method has particular advantages and the combination used varies from group to group. This variety adds a layer of complication because the optimum labelling conditions and labelling efficiency of a given precursor can vary depending on the combination applied (Seemann et al. 2015a). A precursor which can be labelled reliably using any combination of the available technology is desirable, and may facilitate easier translation of labelling between institutions. The labelling experiments have been performed using an Obninsk (EZAG) generator eluted with 0.1 M HCl. The setup is intended to serve as an example to demonstrate the versatility of labelling procedures which can be applied to DATATOC. Further validation of any kit-type labelling protocol requires implementation with a market authorised 68Ga-generator. Radiolabelling of DATATOC was evaluated at ambient temperature using 68Ga PP by each of the four methods, optimised in terms of the labelling pH. Remarkably, RCYs > 95 % were achieved in only 1 min with fractionated and acetone-PP 68Ga, and > 98 % in 10 min regardless of the PP method applied. The precursor concentration increases along the series NaCl-, ethanol-, acetone- and fractionation PP, and is most likely the reason for the increasing rate of labelling along the same series. According to European Pharmacopeia governing 68Ga-DOTATOC a radiochemical purity of at least 91 % is required (measured by HPLC and TLC) prior to in vivo administration, which may require the inclusion of a post-labelling purification (Virgolini et al. 2010). Based on the results gathered such a procedure to improve the RCY is redundant for the preparation of 68Ga-DATATOC. This is analogous to the very successful 99mTc-kits and not only simplifies the process but also saves time – a valuable commodity considering the short half-life of 68Ga compared to 99mTc and 18F.
The influence of lower precursor concentrations has not been tested, but based on the radiolabelling profile it is apparent that there is scope for reduction. Nevertheless, the concentration used compares well with other 68Ga-labelled peptide preparations (Notni et al. 2011; Berry et al. 2011; Lin et al. 2013; Eisenwiener et al. 2002; Dumont et al. 2011; Fani et al. 2011; Ugur et al. 2002; Virgolini et al. 2010; Wester et al. 1997). Based on the results it is evident that the favourable properties of the original DATAM chelator have not been negatively affected to any significant extent by conjugation to TOC. Surprisingly, with NaCl- and ethanol-PP 68Ga, DATATOC shows superior labelling kinetics to the unfunctionalised chelator – DATAM (Seemann et al. 2015b).
There is no evidence for radiolysis of 68Ga-DATATOC on radio-HPLC following labelling. In human serum there was no appreciable release of 68Ga over 2 h, indicating a high metabolic and kinetic stability (98.7 %).
The relative lipophilicities 68Ga-DATATOC and 68Ga-DOTATOC determined by radio-HPLC and shake-flask methods are consistent and show that the former is marginally more lipophilic. Publications involving 68Ga-labelled TOC with different chelators have highlighted the importance of the chelator in terms of in vivo performance, suggesting that the BFC could be tailored to the TV used (Lin et al. 2013; Eisenwiener et al. 2002; Dumont et al. 2011; Fani et al. 2011; Ugur et al. 2002; Wester et al. 1997). Previous work has shown that is possible to modify the lipophilicity of DATA chelators without disturbing the radiolabelling characteristics, offering the potential to look at this relationship more closely (Seemann et al. 2015b).
The feasibility of a kit-type formulation has been assessed using fractionated and acetone-PP 68Ga and produced excellent results. Virtually quantitative yields were obtained in less than 10 min (shorter intervals not analysed) from a lyophilised solid formulation with acetone-PP 68Ga. A similar result was achieved with fractionated 68Ga (97 % RCY). However, it was not possible to lyophilise the precursor formulation due to the tendency of ammonia acetate to sublime under the reduced pressure required to lyophilise the labelling media. This issue can be avoided if the ammonium acetate required for labelling is incorporated into the water used to dilute activity prior to labelling rather than as part of the lyophilised solid.
To the best of our knowledge, this is the first example of a 68Ga- radiopharmaceutical that can be prepared from a lyophilised solid at ambient temperature in less than 10 min. There are examples of 68Ga kits requiring elevated temperatures, but we are only aware of a single ambient temperature kit where labelling of a NOTA-peptide conjugate occurred from a pre-dissolved formulation (Mukherjee et al. 2014; Velikyan et al. 2008). Beyond the development of kit-type formulations, these favourable characteristics can also be exploited through the development of previously inaccessible temperature sensitive biomolecules.
It could be argued that the PP procedures currently available do not compliment the advantages of kit-type labelling because they are manual procedures with a sequence of steps. At this stage this part of the manufacturing process then does not correspond to a kit-type preparation. However, the PP protocols are still simple and routine such that there remains substantial benefits to a very simple, fast and reliable labelling method. Future improvements to the generator and elution procedures may lend themselves better to kit-type labelling, but in the meantime there are sophisticated modules capable of post-processing the eluate with minimal input from the user which would benefit from kit-type labelling.
Conclusion
The first DATA bifunctional chelator has been synthesised and conjugated to TOC in a short seven-step synthesis using affordable starting materials. DATATOC displays remarkable radiolabelling characteristics with > 95 % RCYs possible within 1 min at ambient temperature (pH 4.9, 13 nmol) providing a radiotracer with high human serum stability. 68Ga-DATATOC can be efficiently prepared using 68Ga post-processed by the full range of commonly used methods. The labelling protocols are facile, reliable, robust and do not require non-standard equipment and reagents. Initial efforts towards the development of a kit-type formulation analogous to 99mTc have been successful, and are now being shared with other research groups to assess performance in different settings. In vivo and in vitro studies comparing 68Ga-labelled DATATOC, DOTATOC and NODAGATOC are underway.
68Ga-DATA conjugated radiopharmaceuticals may meet the five main definitions of kit-like preparations: A radiopharmaceutical which can be prepared in a (i) sufficient radiochemical yield (ii) from a lyophilised solid (iii) at room temperature (iv) within a short time (v) that does not require post-labelling purification to meet pharmacopeia standards. The radiochemical performance of DATATOC highlights the potential of DATA-conjugates to carry 68Ga-PET into widespread application through the availability of a true ‘kit-type’ formulation, a facile and cost effective synthesis as well as the preparation of previously inaccessible radiotracers.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Change history
26 November 2018
The metadata in the HTML format of these original articles (Eppard et al., 2017; Decristoforo & Patt, 2017; Domnanich et al., 2017; Chan et al., 2017; Jovalekic et al., 2017; Zhang & Villalobos, 2017; Meckel et al., 2017; Li et al., 2017; Colin et al., 2017; Koziorowski et al., 2017; Ooms et al., 2017; Elsinga, 2017; Seemann et al., 2017; Müller et al., 2017; Luurtsema et al., 2017) were published with an incorrect cover date. The correct cover date is December 2016. This does not alter the text of the original articles.
References
Asti M, Iori M, Capponi PC, Rubagotti S, Fraternali A, Versari A. Development of a simple kit-based method for preparation of pharmaceutical-grade 68Ga-DOTATOC. Nucl Med Commun. 2015;36:502–10.
Baranyai Z, Uggeri F, Giovenzana GB, Bényei A, Bruecher E, Aime S. Equilibrium and kinetic properties of the lanthanoids(III) and various divalent metal complexes of the heptadentate ligand AAZTA. Chem Eur J. 2009;15(7):1696–705.
Berry DJ, Ma Y, Ballinger JR, Tavaré R, Koers A, Sunassee K, et al. Efficient bifunctional 68Ga chelators for positron emission tomography: tris(hydroxypyridinone) ligands. Chem Commun. 2011;47(25):7068–70.
Boros E, Ferreira CL, Cawthray JF, Price EW, Patrick BO, Wester DW, et al. Acyclic chelate with ideal properties for 68Ga PET imaging agent elaboration. J Am Chem Soc. 2010;132(44):15726–33.
Boros E, Ferreira CL, Yapp DTT, Gill RK, Price EW, Adam MJ, et al. RGD conjugates of the H2dedpa scaffold: synthesis, labeling and imaging with 68Ga. Nucl Med Biol. 2012;39(6):785–94.
Breeman WAP, Jong M, Blois E, Bernard BF, Konijnenberg M, Krenning EP. Radiolabelling DOTA-peptides with 68Ga. Eur J Nucl Med Mol Imaging. 2005;32(4):478–85.
Buchmann I, Henze M, Engelbrecht S, Eisenhut M, Runz A, Schaefer M, et al. Comparison of 68Ga-DOTATOC PET and 111In-DTPAOC (Octreoscan) SPECT in patients with neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2007;34(10):1617–26.
Du CM, Valko K, Bevan C, Reynolds D, Abraham MH. Rapid Gradient RP-HPLC Method for Lipophilicity Determination: A Solvation Equation Based Comparison with Isocratic Methods. Anal Chem. 1998;70:4228–34.
Dumont RA, Deininger F, Haubner R, Maecke HR, Weber WA, Fani M. Novel 64Cu- and 68Ga-labeled RGD conjugates show improved PET imaging of ανβ3 integrin expression and facile radiosynthesis. J Nucl Med. 2011;52(8):1276–84.
Eder M, Waengler B, Knackmuss S, LeGall F, Little M, Haberkorn U, et al. Tetrafluorophenolate of HBED-CC: a versatile conjugation agent for 68Ga-labeled small recombinant antibodies. Eur J Nucl Med Mol Imaging. 2008;35(10):1878–86.
Eisenwiener K, Prata MIM, Buschmann I, Zhang H, Santos AC, Wenger S, et al. NODAGATOC, a New Chelator-Coupled Somatostatin Analogue Labeled with 67/68Ga and 111In for SPECT, PET, and Targeted Therapeutic Applications of Somatostatin Receptor (hsst2) Expressing Tumors. Bioconjugate Chem. 2002;13(3):530–41.
Eppard E, Wuttke M, Nicodemus PL, Roesch F. Ethanol-Based Post-processing of Generator-Derived 68Ga Toward Kit-Type Preparation of 68Ga-Radiopharmaceuticals. J Nucl Med. 2014;55(6):1023–8.
Fani M, André JP, Maecke HR. 68Ga-PET: a powerful generator-based alternative to cyclotron-based PET radiopharmaceuticals. Contrast Media Mol Imaging. 2008;3(2):67–77.
Fani M, Del Pozzo L, Abiraj K, Mansi R, Tamma ML, Cescato R, et al. PET of somatostatin receptor-positive tumors using 64Cu- and 68Ga-somatostatin antagonists: the chelate makes the difference. J Nucl Med. 2011;52(7):1110–8.
Fani M, Tamma M, Nicolas GP, Lasri E, Medina C, Raynal I, et al. In vivo imaging of folate receptor positive tumor xenografts using novel 68Ga-NODAGA-folate conjugates. Mol Pharm. 2012;9(5):1136–45.
FDA Grants Orphan Drug Designation for 68Ga-DOTATOC. J Nucl Med. 2014;55(1):10 N.
Frilling A, Sotiropoulos GC, Radtke A, Malago M, Bockisch A, Kuehl H, et al. The impact of 68Ga-DOTATOC positron emission tomography/computed tomography on the multimodal management of patients with neuroendocrine tumors. Ann Surg. 2010;252(5):850–6.
Lin M, Welch MJ, Lapi SE. Effects of chelator modifications on 68Ga-labeled [Tyr 3]octreotide conjugates. Mol Imaging Biol. 2013;15(5):606–13.
Manzoni L, Belvisi L, Arosio D, Bartolomeo MP, Bianchi A, Brioschi C, et al. Synthesis of Gd and 68Ga complexes in conjugation with a conformationally optimized RGD sequence as potential MRI and PET tumor-imaging probes. ChemMedChem. 2012;7(6):1084–93.
Mueller D, Klette I, Baum RP, Gottschaldt M, Schultz MK, Breeman WAP. Simplified NaCl based 68Ga concentration and labeling procedure for rapid synthesis of 68Ga radiopharmaceuticals in high radiochemical purity. Bioconjugate Chem. 2012;23(8):1712–7.
Mukherjee A, Pandey U, Chakravarty R, Sarma HD, Dash A. Single vial kit formulation for preparation of PET radiopharmaceutical: 68Ga-DOTA-TOC. J Radioanal Nucl Chem. 2014;302(3):1253–8.
Notni J, Šimeček J, Hermann P, Wester H. TRAP, a powerful and versatile framework for 68Ga radiopharmaceuticals. Chem Eur J. 2011;17(52):14718–22.
Notni J, Pohle K, Wester H. Be spoilt for choice with radiolabelled RGD peptides: preclinical evaluation of 68Ga-TRAP(RGD)3. Nucl Med Biol. 2013;40(1):33–41.
Ocak M, Antretter M, Knopp R, Kunkel F, Petrik M, Bergisadi N, et al. Full automation of 68Ga labelling of DOTA-peptides including cation exchange prepurification. Appl Radiat Isot. 2010;68(2):297–302.
Parker D, Waldron BP. Conformational analysis and synthetic approaches to polydentate perhydro-diazepine ligands for the complexation of gallium(III). Org Biomol Chem. 2013;11(17):2827–38.
Parker D, Waldron BP, Yufit DS. Crystallographic and solution NMR structural analyses of four hexacoordinated gallium(III) complexes based on ligands derived from 6-amino-perhydro-1,4-diazepine. Dalton Trans. 2013;42(22):8001–8.
Roesch F. Past, present and future of 68Ge/68Ga generators. Appl Radiat Isot. 2013;76:24–30.
Seemann J, Eppard E, Waldron BP, Ross TL, Roesch F. Cation exchange-based post-processing of 68Ga-eluate: A comparison of three solvent systems for labelling of DOTATOC, NO2APBP and DATAM. Appl Radiat Isot. 2015;98:54–9.
Seemann J, Waldron BP, Roesch F, Parker D. Approaching ‘kit-type’ labelling with 68Ga: the DATA chelators. Chem Med Chem. 2015;10:1019–26.
Simeček J, Zemek O, Hermann P, Wester H, Notni J. A monoreactive bifunctional triazacyclononane phosphinate chelator with high selectivity for 68Ga. ChemMedChem. 2012;7(8):1375–8.
Smith DL, Breeman WAP, Sims-Mourtada J. The untapped potential of 68Gallium-PET: the next wave of 68Ga-agents. Appl Radiat Isot. 2013;76:14–23.
Tran K, Khan S, Taghizadehasl M, Palazzo F, Frilling A, Todd JF, et al. 68Ga dotatate PET/CT is superior to other imaging modalities in the detection of medullary carcinoma of the thyroid in the presence of high serum calcitonin. Hell J Nucl Med. 2015;18:19–24.
Ugur O, Kothari PJ, Finn RD, Zanzonico P, Ruan S, Guenther I, et al. 66Ga labeled somatostatin analogue DOTA-DPhe-Tyr-octreotide as a potential agent for positron emission tomography imaging and receptor mediated internal radiotherapy of somatostatin receptor positive tumors. Nucl Med Biol. 2002;29:147–57.
Velikyan I, Maecke H, Langstrom B. Convenient preparation of 68Ga-based PET-radiopharmaceuticals at room temperature. Bioconjug. Chem. 2008;19(2):567–73.
Virgolini I, Ambrosini V, Bomanji JB, Baum RP, Fanti S, Gabriel M, et al. Procedure guidelines for PET/CT tumour imaging with 68Ga-DOTA-conjugated peptides: 68Ga-DOTA-TOC, 68Ga-DOTA-NOC, 68Ga-DOTA-TATE. Eur J Nucl Med Mol Imaging. 2010;37(10):2004–10.
Waengler C, Waengler B, Lehner S, Elsner A, Todica A, Bartenstein P, et al. A universally applicable 68Ga-labeling technique for proteins. J Nucl Med. 2011;52(4):586–91.
Waldron BP, Parker D, Burchardt C, Yufit DS, Zimny M, Roesch F. Structure and stability of hexadentate complexes of ligands based on AAZTA for efficient PET labelling with 68Ga. Chem Commun. 2013;49(6):579–81.
Wester H, Brockmann J, Roesch F, Wutz W, Herzog H, Smith-Jones P, et al. PET-pharmacokinetics of 18F-octreotide: A comparison with 67Ga-DFO and 86Y-DTPA-octreotide. Nucl Med Biol. 1997;24(4):275–86.
Zhernosekov KP, Filosofov DV, Baum RP, Aschoff P, Bihl H, Razbash AA, et al. Processing of generator-produced 68Ga for medical application. J Nucl Med. 2007;48(10):1741–8.
Acknowledgments
The authors thank L. Nichele and J-P. Sinnes for contributions towards the synthesis of DATATOC, and the DFG for a grant (WA 3570/1-1) to support the research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
Author J. Seemann declares that she has no conflict of interest. Author B. P. Waldron declares that he has no conflict of interest. Author D. Parker declares that he has no conflict of interest. Author F. Roesch declares that he has no conflict of interest.
Authors’ contributions
DP and BPW conceived DATATOC and the synthetic route. BPW participated in and supervised the organic synthesis. JS and FR designed the radiochemical experiments, labelling conditions and kit-type labelling procedure. JS carried out the radiochemical evaluations. JS and BPW drafted the manuscript. DP and FR critically revised the manuscript for intellectual content and grammar. All authors read and approved the final manuscript.
Additional file
Additional file 1: Figure S1.
RadioTLC for kit-type 68Ga-labelling of DATATOC at 1, 3, 5, 10 and 15 min. Figure S2: Radioactive (gamma) and UV traces for the kit-type labelling of DATATOC with 68Ga. The unlabelled ligand is evident at 9.1 min (confirmed by injection of the free ligand only) and the 68Ga-labelled complex at 13.0 min. Figure S3: Illustrative example of RadioTLC for stability study of 68Ga-DATATOC 30, 60, 90 and 120 min after exposure to human serum. (DOCX 107 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Seemann, J., Waldron, B., Parker, D. et al. DATATOC: a novel conjugate for kit-type 68Ga labelling of TOC at ambient temperature. EJNMMI radiopharm. chem. 1, 4 (2017). https://doi.org/10.1186/s41181-016-0007-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41181-016-0007-3