Abstract
Background
Fetuses with hypoplastic left heart syndrome (HLHS) and intact interatral septum (IS) have a high perinatal mortality due to the impossibility to guarantee oxygenation at birth. The most effective way to manage this condition seems to create an interatrial communication in utero. We describe a case of IS stenting, performed through an alternative technical approach.
Case presentation
A 32 weeks gestation baby presented with HLHS with intact IS. A left side approach was electively planned, targeting a grossly dilated left pulmonary vein. This maneuver, avoiding the direct puncture of a cardiac chamber, allowed to more safely use a larger cannula and to deploy a larger stent. The procedure was uneventful and granted an adequate flow until the delivery.
Conclusions
Elective interatrial septoplasty through a left side approach seems to be promising in terms of safety and efficacy.
Similar content being viewed by others
Background
HLHS (Hypoplastic left heart syndrome) fetuses with intact interatrial sepum (IS) represents a clinical subset with a particularly high mortality, primarily due to an almost unmanageable hemodynamic condition at birth. Resuscitative measures and emergent Norwood procedure are a possible management with a six-month mortality of 83% in the largest series [1, 2]. Even among newborns undergoing early trans-catheter or surgical procedures, mortality reaches 50% at 30 days [3, 4]. In utero balloon septoplasty and IS stenting have been performed in order to allow proper neonatal oxygenation at birth [5]. In the literature, the procedure is accomplished through the right atrium using a small (18 gauge) cannula. Major technical concerns related to this technique have been raised. Firstly, the need for direct right atrial wall puncture portends a not negligible risk of hemopericardium, even using a small cannula. Secondly, the small cannula itself limits the diameter of the stent that can be negotiated throughout the IS. This issue is particularly relevant when the procedure is performed early during pregnancy. Finally, the usual occurrence of a small left atrium (LA) with a thick IS makes the approach from the right side challenging, or sometimes even impossible [6, 7]. Recent preliminary experiences on a fetal lamb model have demonstrated the feasibility of foramen ovale stenting by trans-venous trans-hepatic approach [8].
This is the first report of a 32 weeks fetus with HLHS syndrome and an intact IS who underwent IS stenting, contemplating a new alternative approach targeting the left pulmonary vein (LPV).
Case presentation
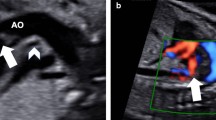
HLHS with intact IS was diagnosed at 32 weeks of gestation, as documented by absence of flow across the septum, and dilated pulmonary veins with a biphasic flow (Fig. 1a). The IS appeared thickened and protruding toward a large right atrium. Owing to this particular anatomy, we planned a procedure of septal stenting from the LPV. The fetus was paralyzed and sedated (IV pancuronium 0.1 mg/kg, fentanyl 30 mcg/kg, atropine 0.02 mg/kg). Under continuous ultrasound guidance, a 15 cm 6 G needle (Cook Medical, Bloomington, IN, USA) was advanced through the fetal thoracic wall and left superior pulmonary vein into the LA. The IS was then punctured after a clear tenting and the tip of the needle advanced in the right atrium. A coronary stent (Multilink Zeta 4 × 15 mm) was advanced over a 0,014″ wire and deployed at 16 atmosphere across the septum.
Panel a Pre procedural echo-colordoppler showing intact interatrial septum, dilated LPV. Panel b Echo-colordoppler at birth showing well-modulated unrestricted flow across the septum. Panel c Doppler signal inside the stent. Panel d Transthoracic echo immediately after birth showing a stable stent across the septum with unrestricted flow. Abbreviations: RA, right atrium; LA, left atrium; LPV, left pulmonary vein
The stent position was confirmed by ultrasounds, and by a marker on the balloon shaft before retracting the balloon and the guide-wire. No sign of fetal suffering, nor of pericardial effusion was observed at the end of the procedure. Immediate post procedural echocardiographic assessment disclosed unrestricted blood flow within the stent, which was maintained until vaginal delivery that was induced at 39 weeks. At birth, no sign of pulmonary hypertension was detected and pulmonary veins displayed a normal Doppler contour (Fig. 1b, c). The stent was properly positioned and completely patent ensuring an unrestricted left to right shunt (Fig. 1d). The baby underwent urgent bilateral banding of pulmonary arteries, owing to significant pulmonary overflow. Upon Norwood stage one procedure, the stent appeared firmly placed across the septum and was easily removed. Thereafter, the child underwent a second stage palliation and, finally, total cavo-pulmonary connection uneventfully.
Discussion
Some degree of IS restriction has been reported to occur in as high as 22% of cases, while a complete obstruction may be demonstrated in 6% [4]. Since the chances of perinatal effective intervention are scant, in utero treatment is being increasingly advocated. The standard procedure is performed by accessing the IS from the right atrium. The left side approach has been performed only in few occasions so far, owing to technical constrains related to the fetal position [5,6,7,8]. The conventional technique displays some challenging steps, in particular the need for puncturing the right atrial wall, which limits the needle and stent diameter (maximum 2.5 mm). Furthermore, the puncture is particularly unfavorable giving the geometric arrangement of the structures, with a very small LA and a thickened septum.
We hypothesized that an alternative approach from the left thoracic wall and through LPV would have obviate most of the aforementioned technical challenges (Fig. 2). By avoiding the direct puncture of the cardiac wall, the risk of significant hemopericardium is reduced. A larger cannula was used, permitting the deployment of a larger stent. Indeed, the 4 mm diameter stent ensured a proper interatrial flow until the delivery. This issue is of pivotal importance whenever the procedure is performed early in fetal life, as in our case. Moreover, the needle tip accommodates in the concavity of the IS and is pushed from a small chamber toward a large chamber, which is mechanically far more favorable.
Conclusions
Although this is an isolated case of IS stenting through the left side approach, the technique demonstrated to be feasible, safe and eventually able to provide the rationale for a more effective and durable procedure. The left side access to the IS with a larger cannula might become the standard of care once its reproducibility on a routine basis has been further verified.
Abbreviations
- HLSH:
-
Hypoplastic left heart syndrome
- IS:
-
Interatral septum
- LA:
-
Left atrium
- LVP:
-
Left pulmonary vein
References
Marshall AC, van der Velde ME, Tworetzky W, Gomez CA, Wilkins-Haug L, Benson CB, Jennings RW, Lock JE. Creation of an atrial septal defect in utero for fetues with hypoplastic left heart syndrome and intect or highly restrictive atrial septum. Circulation. 2004;110:253–8.
Vlahos AP, Lock JE, McElhinney DB, van der Velde ME. Hypoplastic left heart syndrome with intact or highly restrictive atrial septum: outcome after neonatal transcatheter atrial septoplasty. Circulation. 2004;109:2326–30.
Atz AM, Feinstein JA, Jonas RA, Perry SB, Wessel DL. Preoperative management of pulmonary venous hypertension in hypoplastic left heart syndrome with restrictive atrial septal defect. Am J Cardiol. 1999;83:1224–8.
Divanović A, Hor K, Cnota J, Hirsch R, Kinsel-Ziter M, Michelfelder E. Prediction and perinatal management of severely restrictive atrial septum in fetuses with critical left heart obstruction: clinical experience using pulmonary venous Doppler analysis. J Thorac Cardiovasc Surg. 2011;141:988–94.
Bar-Cohen Y, Perry SB, Keane JF, Lock JE. Use of stents to maintain atrial defects and Fontan fenestrations in congenital heart disease. J Interv Cardiol. 2005;18:111–8.
Marshall AC, Levine J, Morash D, Silva V, Lock JE, Benson CB, Wilkins-Haug LE, McElhinney DB, Tworetzky W. Results of in utero atrial septoplasty in fetuses with hypoplastic left heart syndrome. Prenat Diagn. 2008;28:1023–8.
Kalish BT, Tworetzky W, Benson CB, Wilkins-Haug L, Mizrahi-Arnaud A, McElhinney DB, Lock JE, Marshall AC. Technical challenges of atrial septal stent placement in fetuses with hypoplastic left heart syndrome and intact atrial septum. Catheter Cardiovasc Interv. 2014;84:77–85.
Edwards A, Veldman A, Nitsos I, Chan Y, Brew N, Teoh M, Menahem S, Schranz D, Wong FY. Percutaneous fetal cardiac catheterization technique for stenting the foramen ovale in a midgestation lamb model. Circ Cardiovasc Interv. 2015;8:e001967.
Acknowledgements
Not applicable
Funding
None
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Author information
Authors and Affiliations
Contributions
MC and NS performed the procedure described in the case report. PF participated in the study design and coordination. SM and LP helped to draft the manuscript. EC performed the statistical analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
The patient gave her written consent to publish this case report.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ciuffreda, M., Ferrero, P., Ciriello, E. et al. A case report on stent placement in a fetus with hypoplastic left heart syndrome and intact interatrial setptum: first choice alternative standardized approach from left pulmonary vein. J Congenit Heart Dis 2, 1 (2018). https://doi.org/10.1186/s40949-018-0014-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40949-018-0014-1